Journal of HIV for Clinical and Scientific Research
Sonographic appearances of the kidneys and correlation with CD4 count and viral load in adult HIV/AIDS patients in a rural-based infectious disease hospital in sub-saharan Africa
1Department of Radiology, Irrua Specialist Teaching Hospital, Irrua, Nigeria
2Department of Radiology, University of Benin Teaching Hospital, Benin City, Nigeria
3Department of Internal Medicine, Irrua Specialist Teaching Hospital, Irrua, Nigeria
Author and article information
Cite this as
Ehi-Imuse AJ, Adeyekun AA, Irabor PFI, Azubike CO, Izevbekhai SO (2023) Sonographic appearances of the kidneys and correlation with CD4 count and viral load in adult HIV/AIDS patients in a rural-based infectious disease hospital in sub-saharan Africa. J HIV Clin Sci Res. 2023; 10(1): 006-029. Available from: 10.17352/2455-3786.000036
Copyright License
© 2023 Ehi-Imuse AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: The management of HIV infection is often challenging as it can affect every organ in the body including the kidneys. Determination of the Resistivity Indices (RI) of the intra-renal arteries is an emerging non-invasive tool that could predict renal disease.
Aims & objectives: To determine the renal volume, parenchymal pattern, as well as the RI of the intra-renal arteries by Doppler ultrasonography in adult HIV/AIDS patients and correlate findings with CD4 count, viral load and serum creatinine.
Materials & methods: This is a comparative cross-sectional descriptive study that involved sonographic assessment of the renal dimensions, parenchymal echogenicity, and Doppler velocimetry of the segmental intra-renal arteries in 100 apparently healthy confirmed HIV-seronegative control subjects and an equal number of confirmed HIV-seropositive adult patients at Retroviral (RV) clinic of our hospital. A Doppler ultrasound machine with a 3.5MHz Curvilinear probe was used.
Data analysis: Data obtained was analyzed and presented as means which were compared using Student’s t - test, and p values < 0.05 at 95% intervals were considered significant. Pearson’s correlation coefficient was used to assess correlation.
Results: The renal volumes were larger in the HIV/AIDS subjects (right: 125.94 ± 34.02 cm3 and left: 138.99 ± 33.29cm3) than in controls (p = < 0.01) with the left also larger than the right in both HIV/AIDS and control subjects (p = < 0.01, < 0.01) respectively.
There were significantly more individuals with abnormal renal echogenicity in the HIV/AIDS subjects than in the controls.
The RI was significantly greater in the HIV/AIDS than in control subjects.
Both renal volumes showed a weak negative correlation with CD4, viral load, and serum creatinine which was not statistically significant.
There was a statistically significant weak negative correlation between renal echogenicity and CD4 but a positive correlation with viral load and serum creatinine.
RI showed weak negative correlations with serum creatinine and weak positive correlations with CD4 count.
Conclusion: There is a significant difference in renal volume, echogenicity, and RI in HIV/AIDS patients compared to the controls. Renal echogenicity is a better predictor of serum creatinine levels than renal volume and RI. Thus, renal volume and RI do not provide sufficient correlation to be used as a means of monitoring HIV/AIDS patients with renal impairment.
Human Immunodeficiency Virus (HIV) infection and Acquired Immunodeficiency Syndrome (AIDS) are a spectrum of conditions resulting from infection by the human immunodeficiency virus [1-5]. HIV is a retrovirus that causes an immune disorder characterized by a decline of immune function and of Helper T-cells (notably CD4 T cells) [1,5-8]. HIV-infected individuals progress from the asymptomatic stage to that of general lymphadenopathy and then ultimately to AIDS which is clinically characterized by progressive failure of the immune system, life-threatening opportunistic infections, chronic diseases like tuberculosis, cancers, and various multi-systemic pathologies that are rare in individuals with functional immune system [1,5].
The first known case linking HIV/AIDS to renal disease was published in 1984, and it described a case of severe proteinuria with rapid loss of renal function [3]. This was later described as HIV-associated nephropathy (HIVAN) [3]. Since then, the number of HIV-related kidney diseases has increased as the knowledge base widened. Renal manifestations of HIV/AIDS can be classified based on aetiology [3,5,9].
- HIV-specific glomerulopathies; which include HIVAN and a less known HIVICK (HIV Immune complex kidney disease) [10,11].
- Renal disease-related infection resulting from immunosuppression e.g. opportunistic infections like fungal infection, Pneumocystic carinii, and tuberculosis [5].
- Drug-related renal disease particularly antiretroviral nephrotoxicity [12] resulting in Acute Kidney Injury (AKI), Chronic Kidney Disease (CKD), acute interstitial nephritis, Fanconi syndrome, renal tubular acidosis, crystalluria, lithiasis, nephrogenic diabetes insipidus. Also, there are potentially nephrotoxic drugs used in HIV-related opportunistic infections like amphotericin and co-trimoxazole [13].
- Neoplasia causes renal diseases like Kaposis sarcoma and lymphoma [5].
- Vascular causes of renal disease, including renal artery stenosis related to Anti-Retroviral (ARV) drug-induced hyperlipidemia or HIV-related dyslipidemia, hemolytic uremic syndrome, and renal infarction [5].
HIV/AIDS disease progression can be monitored using Plasma HIV RNA level (viral load), Cluster of Differentiation (CD4) lymphocyte counts, serum neopterin levels, serum B2-microglobulin levels, and symptomatic presence of thrush or fever [14]. The viral load is the single best predictor of progression to AIDS and death, followed by CD4+ lymphocyte count and serum neopterin levels [14,15]. A cluster of differentiation (CD4) cell counts, with levels below 200 cells per microliter [16] and viral load greater than 1,000 copies per ml [17,18] are associated with more severity of the disease [16-18]. In clinical research a viral load of 200 copies/ml and below is described as undetectable or untransmittable viral load, while between 200 – 10,000 copies/mL is described as suppressed and about 10,000 copies/mL as unsuppressed [19]. Most HIV-infected patients would develop renal impairment at some point during the disease process which could range from minor transient changes in electrolyte balance to end-stage renal failure [3]. Also due to the increased life expectancy of HIV/AIDS patients there is an increase in the incidence of chronic renal disease which may be due to HIV infection and the adverse effects of Anti-Retroviral Therapy (ART) [3].
Although HIVAN can occur at any stage of the disease, it typically occurs late in the course of HIV-infection, risk factors for its development include a low CD4 count (< 200cells/mm3) and a high viral load [17,18].
Ultrasonography (US) is a safe and convenient imaging modality to demonstrate the increasing renal echogenicity seen in HIVAN [20]. It is affordable, non-invasive, and readily available and does not involve the use of ionizing radiations. Computerized tomography can also be used; however, it is expensive. It involves the use of intravenous contrast and also delivers high radiation to the patients. Magnetic resonance imaging proffers better soft tissue definition of the kidneys than CT, but it is much more expensive and not readily available in our locality [20,21].
The aim of this study was to determine sonographically the renal dimensions and parenchymal pattern as well as the intra-renal Resistivity Index (RI) using Doppler ultrasound in adult HIV/AIDS patients and correlate the findings with CD4 count, viral load, and serum creatinine.
Previous studies have evaluated the usefulness of RI in chronic renal disease especially in Diabetic mellitus (DM) and hypertensive nephropathies. This study has provided information regarding the intra-renal RI in HIV/AIDS patients, its baseline value in individuals without renal impairment, and compared with normal controls.
The findings in this study have also bridged the knowledge gap about the correlation between intra-renal RI in HIV/AIDS patients and serological markers like CD4 count, viral load, and serum creatinine levels. The possibility of RI being used as a non-invasive method of assessing renal involvement in HIV/AIDS patients with renal dysfunction was explored.
Previous studies had correlated CD4 count with renal sonographic findings, however, the emergency of viral load as a monitoring tool; this study correlated data on viral load with renal sonographic and Doppler findings.
HIV/AIDS is a chronic, communicable multi-systemic disease that affects the kidneys as well as other organs in the body including the brain, heart, liver, and eye. As a global pandemic; it has had a lasting and profound impact on our society not only in terms of morbidity and mortality but also serves as a fulcrum of social stigmatization.
HIV/AIDS incidence amongst the young population due to its commonest mode of transmission has helped to maintain its relevance. HIV-infected individuals of African descent are predisposed to renal involvement and ESRD. Direct infection of the renal epithelium by the virus and/or nephrotoxic antiretroviral drugs have been implicated in HIVAN. Ultimately, the resultant renal epithelial disease may lead to changes in the renal parenchyma, renal volume, and possibly vascular resistance. These changes can be assessed with radiological imaging and would help in their early detection before clinical presentation of renal failure.
Ultrasonography is the preferred, commonest, and simplest imaging modality to demonstrate these renal changes. It is affordable, non-invasive, readily available, and does not involve the use of ionizing radiation. High-dose ionizing radiation and cost discourage the use of Computed Tomography (CT). Magnetic Resonance Imaging (MRI) which does not utilize ionizing radiation, provides better soft tissue resolution and capacity for functional study but it is less available and much more expensive.
Studies of renal Doppler ultrasound with assessment of the renal size, parenchyma echogenicity, and vascular velocimetry in HIV/AIDS patients are scanty in our environment. In particular, previous studies on RI were on DM and hypertensive nephropathies; while correlative renal studies were more on CD4 and serum creatinine. The results of this study will contribute to local data on renal Doppler ultrasonographic findings in HIV/AIDS in our setting, and in particular correlation of renal RI with HIV viral load.
Materials and methods
Study design
This was a prospective comparative cross-sectional descriptive ultrasound study. It involved grey-scale sonographic assessment of the renal dimensions, parenchymal changes, and Doppler interrogation of the segmental intra-renal arteries in 100 control subjects and an equal number of confirmed HIV-seropositive adult patients (with laboratory results of their CD4 count and viral load) at ARV clinic in the Irrua Specialist Teaching Hospital, Irrua.
Study location
Irrua is the administrative headquarters of Esan Central Local Government Area (ECLGA) of Edo State which consists of an area of 253 km2 and a population of 105,310 at the 2006 census. ISTH is a tertiary health care facility located in Irrua that services Edo and the neighboring states of Kogi, Delta, and Ondo. Patients are usually referred from general hospitals, government-owned health centers, private hospitals, and other departments in the hospital.
Study population
The study was conducted among adult patients with confirmed HIV infection attending the ARV clinic at the ISTH, Irrua, Edo State, Nigeria. The estimated population of HIV/AIDS patients actively attending this clinic is one thousand one hundred and fifteen individuals.
Inclusion criteria for study subjects
Confirmed HIV seropositive adult patients attending the ARV clinic of the Irrua Specialist Teaching Hospital (ISTH). They included:
- ARV clinic patients whose HIV infection has been confirmed following voluntary counseling and testing using the National HIV screening Algorithm II.
- Patients with CD4 count result within 6 months of recruitment.
- Patients with viral load result within 6 months of recruitment.
- Patients who gave written informed consent.
- Patients aged 18 years and above.
Exclusion criteria for study subjects
- Patients less than 18 years of age
- Patients who refused to give informed consent.
- Patients with a past medical history of hypertension, diabetes mellitus, or malignancies.
- Patients with known pre-existing renal disease.
- Patients with obstructive uropathy evidenced by hydronephrosis on ultrasound scan.
Inclusion criteria for study controls
Subjects were confirmed HIV seronegative adults who presented voluntarily to the HIV Testing Services office (HTS), ISTH for Voluntary Counseling and Testing, and whose status had been properly communicated to them by HTS counselors. Control subjects shall be matched for age and sex with study subjects. They were also:
- Individuals who have been confirmed negative for HIV infection following voluntary counseling and testing using the National HIV screening Algorithm II.
- Individuals without any known history of Diabetes mellitus or hypertensive.
- Individuals who are normotensive following BP check.
- Individuals who give written informed consent.
- Individuals from 18 years and above.
Exclusion criteria for study controls
- Individuals aged less than 18.
- Individuals who refused to give consent.
- Individuals with a past history of hypertension, diabetes mellitus, or malignancy.
- Individuals with known renal disease.
- Patients with obstructive uropathy evidenced by hydronephrosis on ultrasound scan.
Sample size determination
The study size was calculated for a cross-sectional study using Cochran’s formula [22,23]. Below, using an adult population HIV/AIDS prevalence rate of 5.5% [24].
Where; Ns = the required sample size
Z = standardized normal deviation = 1.96
P = the estimate of the prevalence of the number of people with HIV in the population in South Southern Nigeria = 5.5%.
D = degree of accuracy set at 0.05
Hence, the sample size is calculated as;
Ns = (39.2)2 (0.055) (0.945)
Ns = (1536.64) (0.051975)
Ns = 80
The sample size value of 80 was approximated to 100 study subjects so as to increase the power of the study and an equal number (100) of control subjects, giving a total sample size of 200 subjects.
Methodology
This prospective study was conducted in an ultrasound room located in Accident and Emergency under the purview of the department of Radiology, in ISTH, Irrua. This is to ensure some level of privacy for the study subjects. All sonograms were done by the researcher under the supervision of the project supervisor to minimize inter-observer error. Ethical approval was granted by the Ethics and Research committee of Irrua Specialist Teaching Hospital. Ethical approval was received before the commencement of the study. Informed written consent was obtained from all subjects and controls.
Clinical evaluation
The socio-demographic data such as age, ethnicity, religion, marital status, occupation, level of education, and sex were documented. Also, past medical and drug history such as the date and duration of confirmation of HIV status, date of commencement and duration of antiretroviral drugs, current antiretroviral drug line (1st or 2nd line), current antiretroviral drug combination with duration of use (computed from date of commencement of ART to study date) were obtained.
Blood pressure was taken using a sphygmomanometer and the systolic and diastolic readings were recorded. The subject’s height and weight were measured and recorded.
BMI was calculated as follows
- Body Mass Index (BMI) [25] = Weight (kg) / Height (m)2
CD4 and viral load are routinely carried out in retroviral clinics every six months for each patient on ARV drugs, and also serum creatinine for patients with suspected renal impairment. The most recent serum creatinine, CD4 counts, and viral load (within 6 months of recruitment) were obtained from the patients’ case notes or the LAMIS (HIV AIDS Electronic Medical Records). In cases where there were no recent readings, patients were sent for routine blood sample collection at the Virology laboratory, venipuncture was performed by laboratory technicians, and the sample was analyzed for serum creatinine.
A normal CD4 cell count ranges from 500 to 1,200 cells/mm3 in adults with good immune systems [26]. The results of the CD4 cell counts in HIV/AIDS subjects were categorized [26] into two groups as follows:
- CD4 cell count less than 200 cells/mm3 is defined as those with immune suppression and are susceptible to opportunistic infections.
- CD4 cell count greater than 200 cells/mm3.
The results of the viral load were categorized into undetectable, suppressed, and unsuppressed groups as follows:
- Undetectable viral load – defined as a viral load below the detectable threshold which is usually less than 200 copies/mL indicates sustained viral suppression
- Suppressed viral load – defined as a viral load between 200 copies/mL and 1,000 copies/mL signifying a stable viral load on ART
- Unsuppressed viral load – defined as viral load above 1,000 copies/mL indicating a virologic failure [26].
Ultrasound evaluation
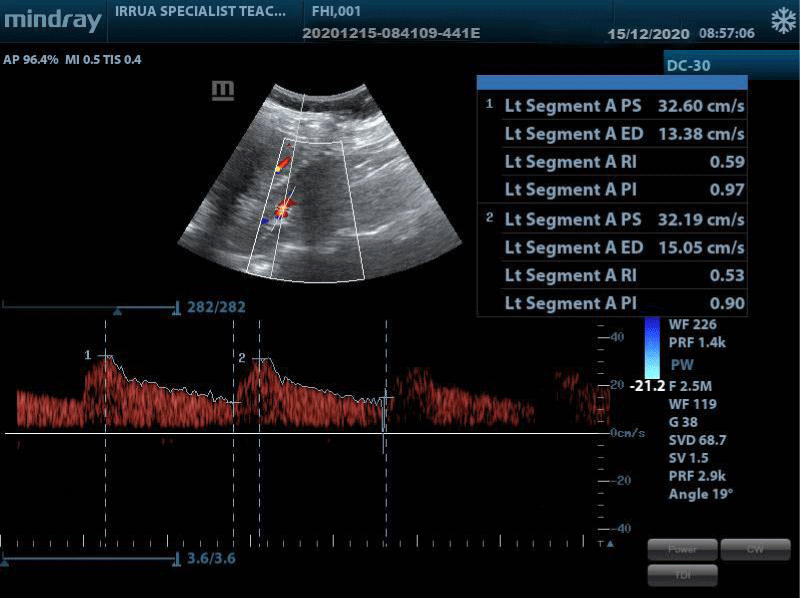
The US examination was performed using a curvilinear probe with a frequency of 3 – 5 MHz of a Mindray DC-30 (Shenzhen Mindray Bio-Medical Electronics Co., Ltd, China 2017) ultrasound machine with color and pulse Doppler facility. Each subject was made to lie supine on the couch with the abdomen adequately exposed from the upper abdomen to the symphysis pubis. The examination was performed with an empty bladder. Coupling gel was applied over the upper abdomen, using the liver on the right and spleen on the left as the acoustic windows. Longitudinal and transverse scans with Doppler interrogation of the kidneys were performed in the supine, supine oblique, and prone positions.
The following parameters were evaluated
1. Renal parenchymal echogenicity: Renal parenchymal echogenicity was assessed on the supine views only and graded using the Hricak, et al. [27] grading given as follows: (Figures 1–4).
- Grade 0: Normal renal cortical echogenicity less than liver and splenic parenchymal echogenicities on the right and left sides respectively with good corticomedullary differentiation.
- Grade 1: Renal cortical equals liver and splenic parenchymal echogenicities on the right and left sides respectively with good corticomedullary differentiation.
- Grade 2: Renal cortical echogenicity is greater than liver and splenic parenchymal echogenicities on the right and left respectively; but less than renal sinus echogenicity with moderate loss of corticomedullary differentiation.
- Grade 3: Renal cortical echogenicity is greater than both liver and splenic parenchymal as well as renal sinus echogenicity with complete loss of corticomedullary differentiation.
2. Renal size: For renal dimensions, images were acquired in the longitudinal plane with both renal poles clearly demonstrated and on the transverse plane at the level of the hilum. Using electronic calipers, the renal sagittal or coronal length (L) was taken as the longest distance between the renal poles while the depth (D) was taken as the widest transverse diameter both on the longitudinal scan (Figure 5) and the renal width (W) as the maximum transverse diameter on the transverse scan (Figure 5). All measurements were taken thrice, with the average recorded to reduce intra-observer variability.
The renal volume was thereafter computed using the formula for an ellipse (L x W x D x π/6) cm3 which is equivalent to (L x W x D x 0.524) cm3 [28].
3. Resistive index: Triplex Doppler sonography was performed and spectral waveforms were obtained from the segmental renal arteries. Relevant measurements were taken from the upper, middle, and lower pole segmental arteries which were insonated using a 2 mm to 4 mm Doppler gate and an angle of insonation (theta) less than 20 degrees to enhance signal quality. The average of the three measurements was calculated and documented as the value for the kidney.
The values of the Peak Systolic Velocity (PSV), End Diastolic Velocity (EDV), Systolic Diastolic ratio (S/D) ratio, Resistivity Index (RI), and Pulsatility Index (PI) were recorded from the machine-generated values using auto-trace function.
The SD ratio is defined as the ratio of the peak systolic and end-diastolic velocities [29].
The Resistivity Index (RI) is derived from the formula [29];
The Pulsatility Index (PI) also known as the Gosling index is calculated using the formula [29];
All the obtained were recorded in the data proforma and analyzed.
Data analysis
Data obtained from the proforma, including sonographic findings, were entered into a Microsoft Excel Spreadsheet and analyzed using IBM SPSS Statistics for windows, version 20.0. Armonk, NK: IBM Corp [30].
Data comparison (statistical test of significance) was done using Spearman or Pearson Correlation analysis, the chi- square test for categorical data, and the student t - test and ANOVA (Analysis of Variance), where applicable. At a 95% confidence interval, two-tailed p - values less than or equal to 0.05 were considered statistically significant.
Limitations
- B-mode and Doppler sonographic assessment of the left kidney may be difficult in some patients especially the obese due to poor visualization; and in severely ill patients due to difficulty in performing breath holding and remaining still during insonation of the renal artery. This can be overcome but may prolong the scan time.
- Ultrasonography is operator-dependent, therefore variations in measurement may occur, but this will be minimized by taking an average of three (3) measurements for each dimension of the kidney and three (3) for Doppler parameters to minimize intra-observer error. Also, all the scans and data importation will be done by the researcher to minimize inter-observer errors.
Results
Two hundred subjects consisting of 100 laboratory-confirmed HIV-seropositive patients as well as an equal number of age and sex-matched apparently healthy adults as controls participated in this study. Both right and left kidneys were assessed for each subject; a total of 200 kidneys were assessed for the HIV/AIDS subjects and 200 kidneys for the control subjects. Hence, a total of 400 kidneys were assessed in this study.
Socio-demographics
Table 1 shows the socio-demographic parameters of the study population. The age range of the study population was from 17–77 years with a mean age of 41.4 ± 11.6 years and 41.3 ± 11.5 years for the HIV/AIDS patients and controls respectively. The difference in age range was not statistically significant; (p = 1.00). The modal age group was 39 – 58 years accounting for 51 (51.0%) of both subjects and controls, there was also no significant statistical difference (p = 1.00).
The HIV/AIDS subjects and apparently healthy controls comprised 34 (34.0%) males and 66 (66.0%) females, the male-to-female ratio was 1:1.9. There was no significant statistical difference in the sex distribution of the study population (p = 1.00).
The ethnic distribution of the HIV/AIDS and control populations showed significant statistical difference (p = < 0.001); with the Esan ethnic group constituting 74 (74.0%) and 60 (60.0%) of the HIV/AIDS and control subjects respectively. This was followed by Etsako comprising 15 (15.0%) of study subjects and Ibo comprising 22 (22.0%) in the controls.
The religion distribution of the HIV/AIDS and control subjects showed a significant statistical difference (p = 0.005); with more Muslims in the HIV/AIDS subjects 14 (14.0%) compared with 3 (3.0%) for the control subjects. There are also more Christians in the control subject comprising 97 (97.0%) compared with 86 (86.0%) in the HIV/AIDS subjects.
Most of the study subjects were married, 65 (65.0%) for the HIV/AIDS compared with 75 (75.0%) for the control subjects. While 12 (12.0%) and 2 (2.0%) of the HIV/AIDS subjects are divorced and widowed respectively, none was recorded for the control subjects. The distribution of marital status between HIV and control subjects showed a statistically significant difference (p < 0.001).
The occupations of the subjects in the HIV/AIDS compared with those in the control showed a statistically significant difference (p < 0.001) with more self-employed artisans 56 (56.0%) among the HIV/AIDS subjects than 45 (45.0%) in the control subjects. While there were more Professionals and students 26 (26.0%) and 20 (20.0%) in the control subject compared with 3 (3.0%) and 9 (9.0%) in the HIV/AIDS subjects.
There was a statistically significant difference (p < 0.01) in the level of education between the HIV/AIDS and control subjects with 72 (72.0%) of the control having tertiary education compared with 48 (48.0%) in the HIV/AIDS subjects. Also, more of the HIV/AIDS subjects 16 (16.0%) and 35 (35.0%) had primary and secondary education compared with 3 (3.0%) and 25 (25.0%) respectively for the control subjects.
Duration of disease and ARV drug usage
Table 2 shows the durations of retroviral diagnosis, overall ART usage, and current ART usage in HIV/AIDS subjects. The duration of retroviral diagnosis ranged from 1 to 15 years with a mean value of 5.77 ± 4.12 years. The majority of the study subjects (50%) were diagnosed less than 5 years ago with a mean (SD) value of 2.06 ± 1.316 years.
All HIV/AIDS subjects were on ART for a mean duration of 5.07 ± 3.85 years. Most of the study subjects (55.0%) are within the less than 5 years group with a mean duration of 1.96 ± 1.33 years. There was a statistically significant difference between the duration of retroviral diagnosis and duration of ART use; p = < 0.01; this implies that most of the study subjects did not start ART immediately after their diagnosis was made.
Table 3 shows the current ART type and mean duration of use. About 96 (96.0%) of subjects are currently on first-line ART with a mean duration of 2.1 years. Also, 75 (75.0%) of the subjects are currently using TDF-3TC-DTG combination with a mean duration of 2.3 years while 11 (11.0%) of the subjects are currently using ABC-3TC-DTG combination. Also, the second-line drugs that contain protease inhibitors (ATVr and LPVr) were in use by 4% of the HIV/AIDS patients.
Anthropometric indices
The Comparison of anthropometric indices in HIV/AIDS and control subjects is shown in Table 4. The mean weight, height, and BMI were 67.27 ± 16.04kg, 1.57 ± 0.12m, and 27.24 ± 6.01 for the HIV/AIDS subjects while the controls were 72.07 ± 15.43kg, 1.56 ± 0.24m and 32.73 ± 15.97. All parameters of the controls were greater than that of the HIV/AIDS subjects. However, only differences in the mean weights and BMI between the HIV/AIDS and control subjects were statistically significant; p = 0.03 and < 0.01 respectively.
Laboratory evaluation
The HIV/AIDS subjects had a mean CD4 cell count of 433.66 ± 339.33 cells/µL (range: 18 - 1,920 cells/µL). The mean values were above the immunosuppression levels characterized by a CD4 cell count of less than 200 cells/µL. The CD4 cell count was slightly higher in females 438.65 ± 314.479 cells/µL than in males 423.97 ± 387.92cells/µL but this difference was not statistically significant (Table 5).
Patients with immunosuppression defined as CD4 cell count less than/or equal to 200 cells/µL were significantly lower 23 (23.0%) than those with CD4 cell count above 200 cells/µL 67 (67.0%); p = < 0.01 (Table 6).
The mean viral load for the HIV/AIDS population was 539,000.65 ± 183,133.86 copies/mL with a range of 0 to 1,250,000 copies/mL. The mean viral load was higher in males with 80,606.56 ± 248,801.68 copies/ml than in female subjects with 40,143.06 ± 138,160.84 copies/ml; p = 0.085 but the difference was not statistically significant (Table 5). More than half (51.0%) had unsuppressed viral loads and forty-five (45.0%) had undetectable viral loads which were significantly higher than 4 (4.0%) subjects with suppressed viral load p = < 0.01 (Table 6).
The HIV/AIDS subject had a mean serum creatinine of 98.35 ± 106.9mmol/dl with a range of 35.37mmol/dl to 831.15mmol/dl. The mean and range of serum creatinine in the HIV/AIDS subjects were significantly higher than in the control subjects who had a mean serum creatinine of 74.80 ± 25.27mmol/dl with a range of 17.68mmol/dl to 150.31mmol/dl; p = 0.033 (Table 7).
Ultrasound evaluation
Renal parenchymal echogenicity: Table 8 shows the patterns of renal parenchymal echogenicity for the right and left kidneys. Of all the control subjects 100 (100.0%) had grade 0 normal parenchymal echogenicity, compared with 71 (71.0%) in the HIV/AIDS subjects. However, 29 (29.0%) of the HIV/AIDS subjects showed abnormal echogenicity with grade I being the most prevalent, 18 (18.0%); followed by grade III, 6 (6.0%), and grade II with 5 (5.0%). There was a statistically significant difference in renal parenchymal echogenicity between the HIV/AIDS patients and controls respectively; p < 0.01.
Both right and left kidneys had the same distribution in renal parenchymal echogenicity in the HIV/AIDS and control subjects. There was no statistically significant difference in the parenchymal echogenicity between the right and left kidney for both HIV/AIDS and control subjects respectively; p < 0.99.
Renal dimensions: Table 9 shows a comparison of renal dimensions between the HIV/AIDS subjects and controls. The mean right renal length, breadth, height, and volume in the HIV/AIDS subject were 10.31 ± 0.96cm, 5.53 ± 0.75cm, 4.40 ± 0.51cm and 125.94 ± 34.02cm3 respectively. While the mean left renal length, breadth, height, and volume in the HIV/AIDS subject measured 10.61 ± 0.87cm, 5.61 ± 0.63cm, 4.60 ± 0.45cm and 138.99 ± 33.29cm3 respectively. All the measured left renal dimensions were greater than those of the right kidney; however, only the left renal length, height, and volume showed a statistically significant difference compared with the right; p - values < 0.001.
For the controls, the right renal length, breadth, height, and volume measured 9.96 ± 0.76cm, 5.26 ± 0.54cm, 4.07 ± 0.49cm and 111.82 ± 25.36cm3 respectively. While the left renal length breadth, height, and volume in the controls measured 10.15 ± 0.96cm, 5.38 ± 0.96cm, 4.32 ± 0.51cm and 126.52 ± 35.81cm3 respectively. Just like in the HIV/AIDS subjects, the left renal dimensions were greater than those of the right kidney; however, only the left renal length, height, and volume showed statistically significant differences when compared with the right; p - values < 0.001. The difference in side-to-side renal breadths in both groups was not statistically significant.
The mean values of all the renal parameters were statistically significantly higher in HIV/AIDS subjects compared with the controls with p - values for the right renal length, breadth, height, and volume at p = < 0.01, < 0.01, < 0.01 and < 0.01 respectively, while p-values of the left renal length, breadth, height, and volume are p = < 0.01, < 0.01, < 0.01 and 0.01 respectively.
Renal doppler parameters: Table 10 shows a comparison of renal Doppler velocimetry between HIV/AIDS and control subjects. In HIV/AIDS patients, the right renal PSV, EDV, SD, RI, and PI were greater than those for the left, except for the EDV, measuring 37.55 ± 8.03cm/s, 14.03 ± 8.50cm/s, 2.69 ± 0.32cm/s, 0.63 ± 0.04 and 1.10 ± 0.16 respectively versus 37.38 ± 12.40cm/s, 14.96 ± 5.33cm/s, 2.56 ± 0.34cm/s, 0.60 ± 0.05 and 1.02 ± 0.18 for the left renal values respectively. However, only the S/D, RI, and PI showed significant statistical differences between the right and left kidneys; p – values < 0.01, < 0.01 and < 0.01 respectively.
For the controls, the right renal PSV, EDV, S/D, RI, and PI in the controls measured 31.30 ± 6.72cm/s, 13.10 ± 3.18cm/s, 2.48 ± 0.39cm/s, 0.59 ± 0.06 and 0.97 ± 0.19 respectively. The left renal PSV, EDV, S/D, RI and PI in the controls measured 33.35 ± 9.57cm/s, 14.0 ± 3.89cm/s, 2.40 ± 0.33cm/s, 0.58 ± 0.05 and 0.94 ± 0.21 respectively. The right S/D, RI, and PI were greater than the left but only the S/D and RI were statistically significant; p - values 0.003 and 0.004 respectively. The left PSV and EDV were greater than the right and this difference was not statistically significant; p - values 0.09 and 0.06 respectively.
All the mean renal artery Doppler parameters (PSV, EDV, S/D, RI, and PI) of both kidneys were higher in the HIV/AIDS patients compared with the controls. There was a statistically significant difference in the right renal PSV, EDV, S/D, RI, and PI between the HIV/AIDS and control subjects; p = < 0.001, 0.038, < 0.001, < 0.001, and < 0.001. There was also a statistically significant difference in the left renal PSV, S/D, RI, and PI between HIV/AIDS and control subjects; p = 0.011, 0.001, <0.001, and 0.004. However, only the left renal EDV did not show any statistically significant difference; p –value 0.146 between the HIV/AIDS and control subjects Figure 6.
Correlations in hiv/aids patients
Table 11 shows the correlation matrix of the relationship between Laboratory and Ultrasound parameters of the right kidney in HIV/AIDS patients; while Table 12 shows the correlation matrix of the relationship between Laboratory and Ultrasound parameters of the left kidney in HIV/AIDS patients.
CD4 count with viral load and serum creatinine
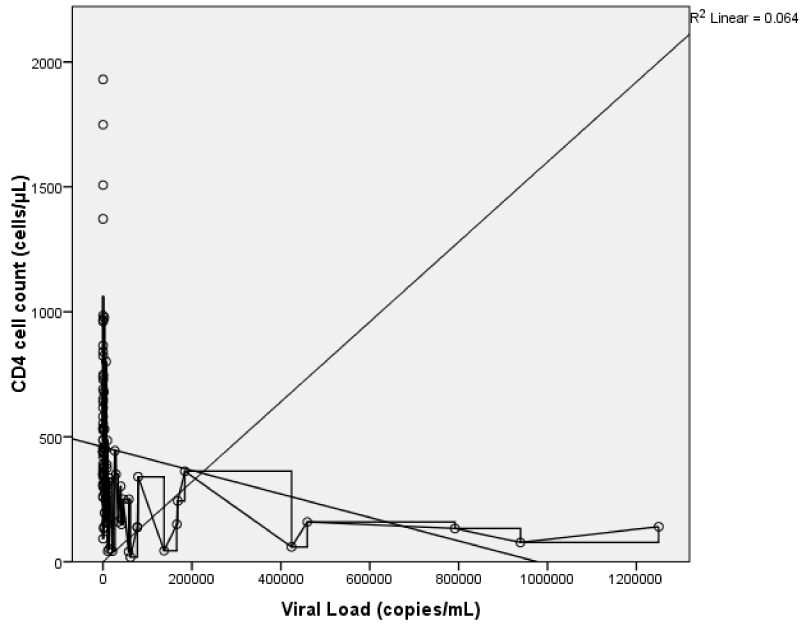
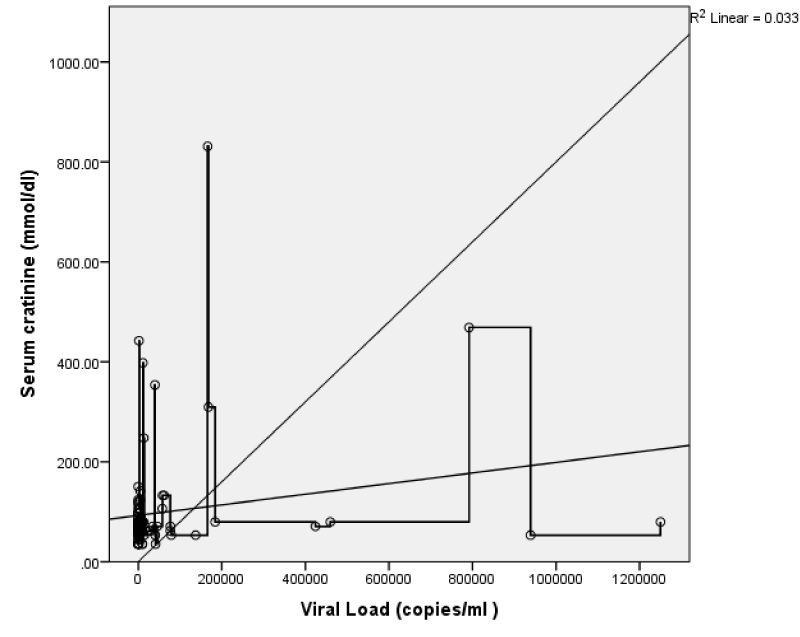
The CD4 count showed a weak but statistically significant negative correlation with viral load; r = -0.25, p = < 0.01 (Figure 7) and serum creatinine; r = - 0.19, p = 0.03. This implies that as the CD4 value increases both the viral load and serum creatinine values showed statistically significant decreases. There was also a weak but statistically significant positive correlation between viral load and serum creatinine; r = 0.18, p 0.04 (Figure 8). This implies that as viral load increases, there is an increase in serum creatinine.
Renal dimensions with CD4, viral load, and serum creatinine
The right and left renal volumes showed non statistically significant, weak negative correlation with CD4 count (r = -0.08, -0.12; p = 0.23, 0.11) (Figure 9). This implies that as the CD4 count increases the right and left renal volumes reduces slightly but not statistically significant. Also, the left renal length, breadth, height, and right renal length showed a weak negative correlation with CD4 count, and only left renal length and height being statistically significant. While the right renal breadth and height did not show any statistically significant correlation with CD4 count (Figure 9).
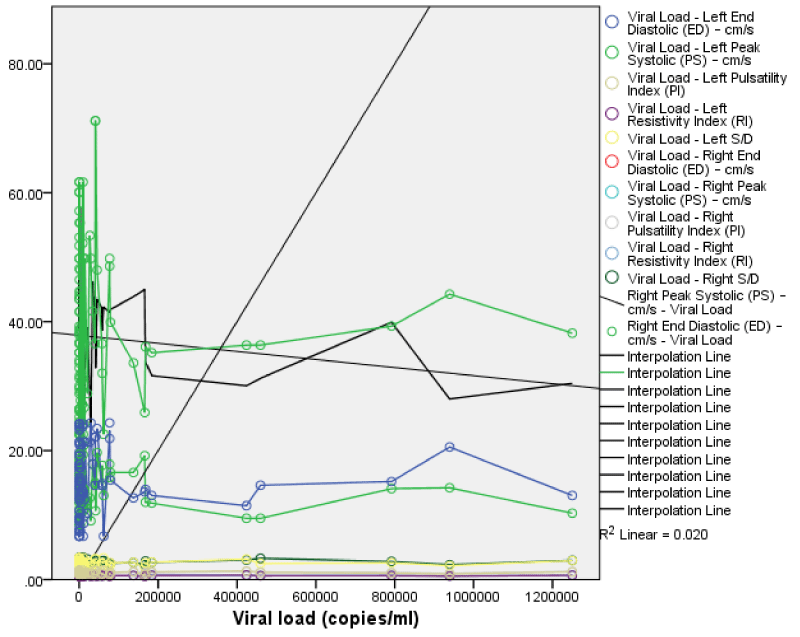
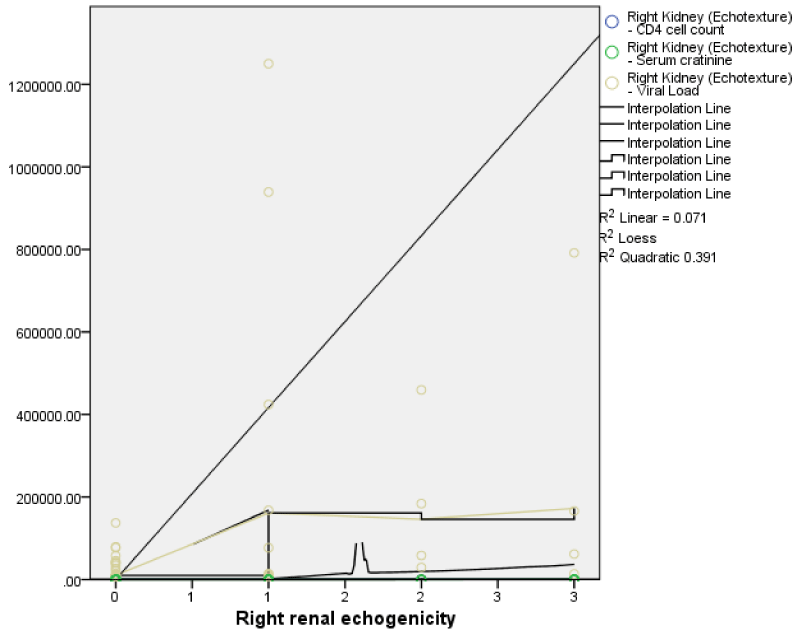
The viral load showed a weak negative but not statistically significant correlation with volumes and dimensions of the right and left kidneys with the exception of the left renal length which showed a weak positive statistically insignificant correlation with viral load. (Figure 10). This implies that as the viral load increased, the right and left renal volumes and almost all the renal dimensions showed a decrease in measured values Figures 7-11.
The serum creatinine showed a weak negative correlation with right and left renal volumes which was not statistically significant (r = -0.12, -0.12; p = 0.13, 0.42). This implies that with an increase in the serum creatinine values, there is a decrease in the right and left kidney volumes which was not statistically significant.
Renal parenchymal echogenicity with viral load, CD4
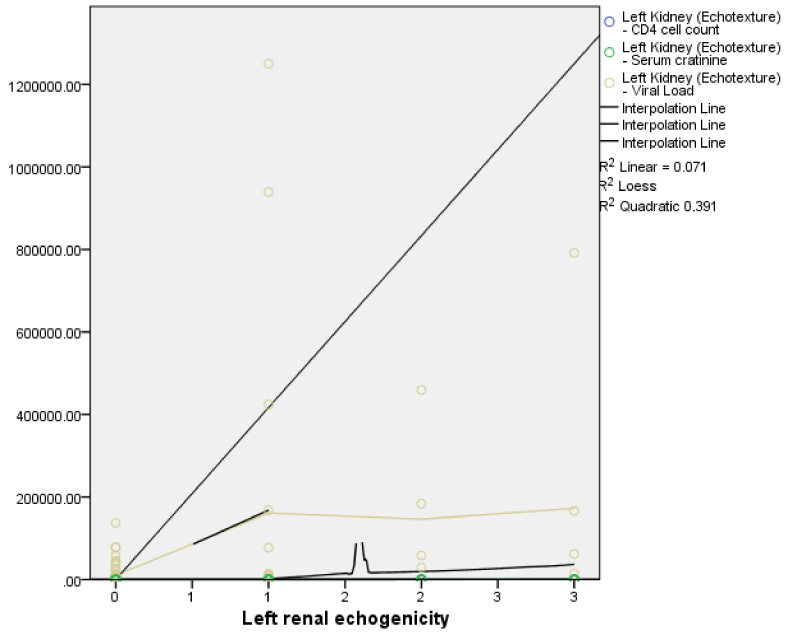
There was a positive statistically significant correlation between right renal echogenicity and viral load (r = 0.33, p = < 0.01), as well as between right renal echogenicity and serum creatinine (r = 0.50, p = < 0.01). There was also a negative statistically significant correlation between right renal echogenicity and CD4 count (r = -0.27, p = < 0.01). These findings were similar for the left kidney with a positive significant correlation between left renal echogenicity and viral load (r = 0.33; p = < 0.01), as well as between left renal echogenicity and serum creatinine (r = 0.50, p = < 0.01). There was also a negative statistically significant correlation between left renal echogenicity and CD4 count (r = -0.27, p = < 0.01).
This implies that with an increase in serum creatinine and viral load, there was a corresponding statistically significant increase in the grades of both right and left renal echogenicity. Also, an increase in CD4 count corresponds with a decrease in the grades of both right and left renal echogenicity.
Renal doppler parameters with CD4, Viral Load, and serum creatinine
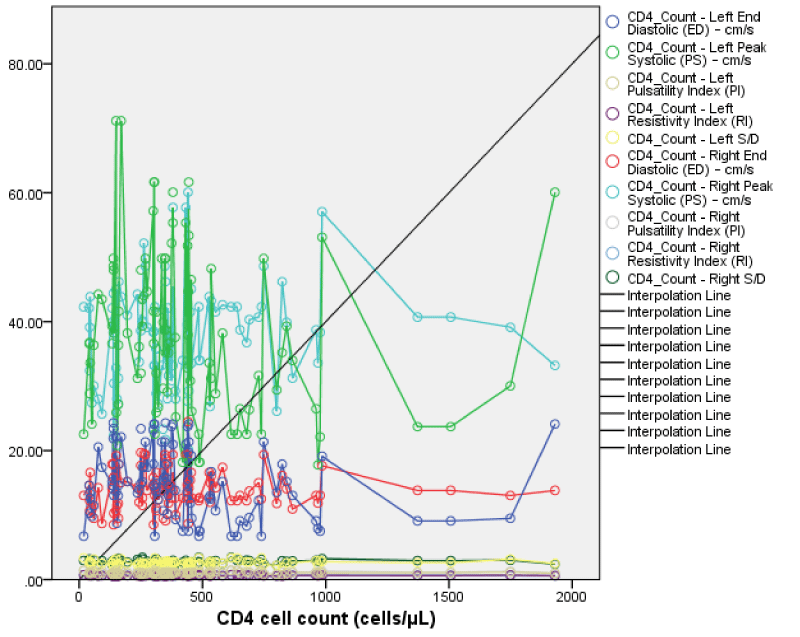
The CD4 count showed a positive but not statistically significant correlation with right renal PSV (r = 0.12, p = 0.12), EDV (r =0.02, p =0.43), S/D (r = 0.15, p = 0.07), RI (r = 0.11, p = 0.14) and PI (r = 0.04, p 0.34). This implies that as the CD4 count increased there was some increase in the right renal Doppler parameters; although this increase was not statistically significant Figures 12-15.
The CD4 count showed a weakly positive correlation between CD4 count and left S/D, RI, and PI (r = 0.16, 0.17, and 0.09; p = 0.06, 0.04, and 0.19) which is statistically significant for only the left RI. However, mean left PSV and EDV showed a weakly negative correlation with CD4 (r = - 0.16, - 0.19; p = 0.06, 0.03) which was significant for EDV. This implies that as the CD4 count increases most of the Doppler parameters (Left S/D, RI, and PI) increased and only left RI was statistically significant. The others left PSV and EDV reduced with increased CD4.
Comparing the correlation of CD4 count with the left and right Doppler parameters; most showed a positive correlation with CD4 although not significant except for left RI. This implies that both RI and most of the right renal parameters increased with an increase in the CD4 count and only the increase in left RI being statistically significant.
The viral load showed a negative but non statistically significant correlation with right PSV (r = -0.14, p = 0.08), and EDV (r = -0.01, p = 0.10). It showed a positive but not statistically significant correlation with right renal S/D (r = 0.07, p = 0.26), RI (r = 0.03, p = 0.40), and PI (r = 0.01, p = 0.45). This implies as the viral load increases there appears to be a decrease in the PSV and EDV while the S/D, RI, and PI appeared to increase; but these changes where not statistically significant.
The viral load also showed weak positive but not statistically significant correlation with left renal PSV, EDV, S/D, RI, and PI (r = 0.04, 0.03, 0.04, 0.04, 0.06; p = 0.34, 0.40, 0.34, 0.36, 0.29). This implies that as viral load increased there was also an increase in the left renal Doppler parameters but not statistically significant. Comparing the viral load with left and right Doppler parameters; there is a weak positive correlation which was not statistically significant.
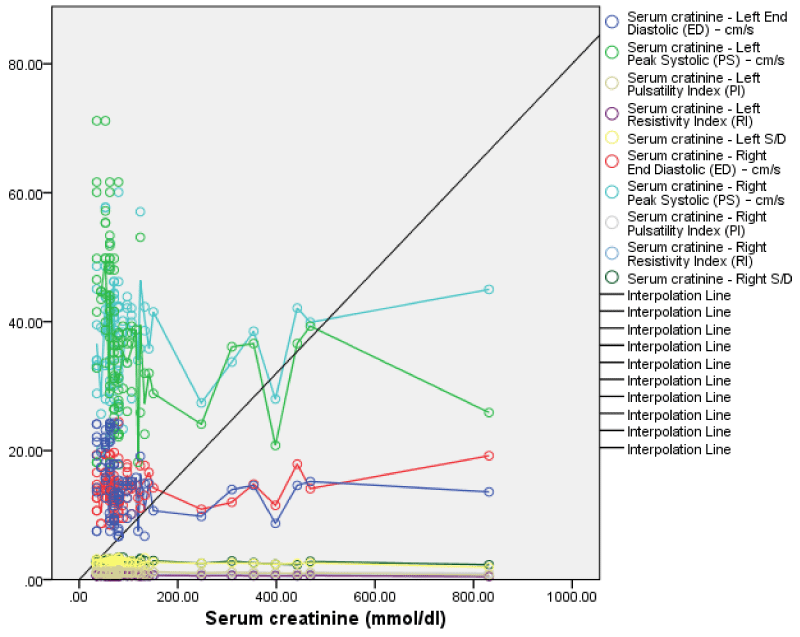
The serum creatinine also showed a weak negative but non-statistically significant correlation with right renal S/D (r = -0.12, p = 0.11), RI (r = -0.12, p = 0.11) and PI (r = -0.11, p = 0.13). While, PSV (r = 0.05, p = 0.30) and EDV (r = 0.12, p = 0.12) showed weak positive but not significant correlation (Figure 15). This implies that as the serum creatinine increases there was some decrease in the S/D, RI, and PI while the PSV and EDV showed some increase; both changes were however not statistically significant.
The serum creatinine showed a weakly negative correlation with left renal PSV, EDV, S/D, RI, and PI (r = -0.21, -0.15, -0.15, - 0.18, -0.15; p = 0.02, 0.06, 0.07, 0.03, 0.06); which is significant for only the left renal RI (Figure 2). This implies that as the serum creatinine increases the left renal PSV, EDV, S/D, RI, and PI all decreased with only changes in RI being statistically significant. Comparing the serum creatinine with the right and left RI, PSV, EDV, and S/D both showed a negative correlation, and the left renal RI was statistically significant.
Renal dimensions with renal doppler parameters
There was a weak negative but not significant correlation between right renal volume and PSV (r = -< 0.01, p = 0.49), S/D (r =-0.03 p = 0.40), RI (r = -0.05, p = 0.32) and PI (r = -0.02, p = 0.23). This implies that with an increase in renal volume the right PSV, S/D, RI, and PI showed some decrease in value which was not statistically significant.
The left renal RI shows a negative correlation with left renal volume (r = -0.01; p = 0.46). This implies that as the left renal RI increases the renal volume decreases. Comparing right and left renal RI with renal volume; unlike the left kidney, the right renal RI shows a positive correlation with right renal volume Figures 1,2.
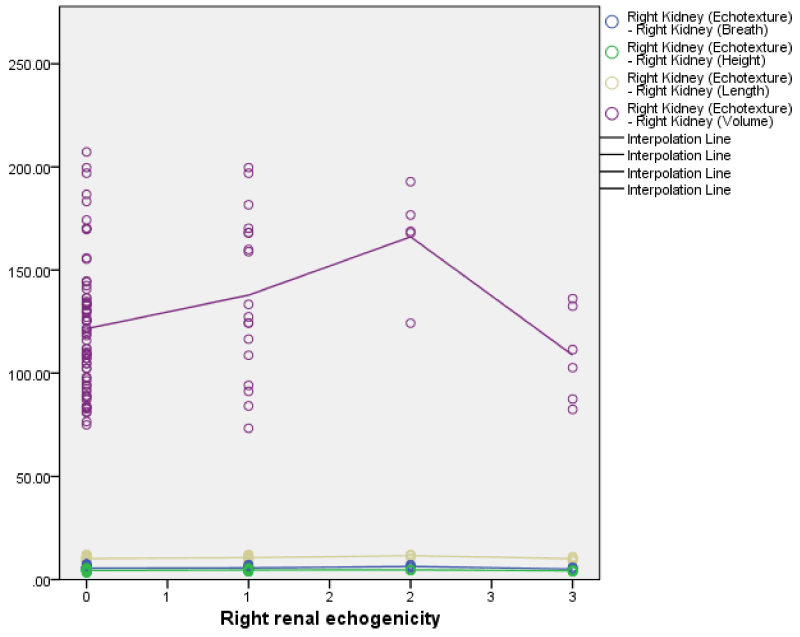
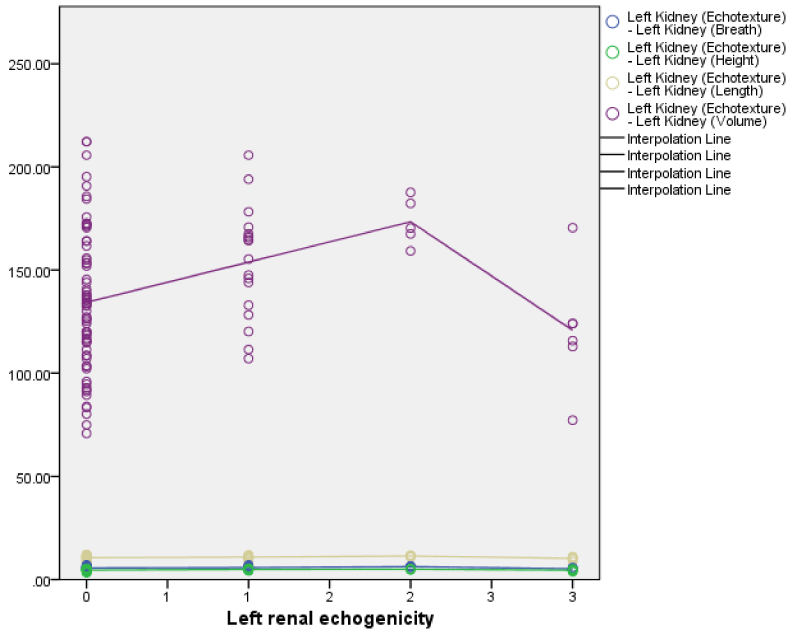
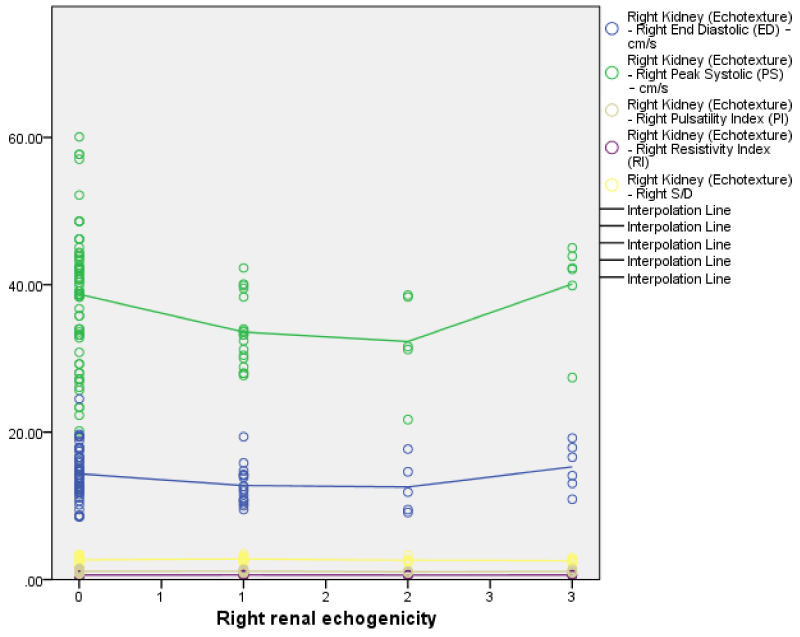
The right renal echogenicity showed a weakly positive correlation with right renal length, breadth, height, and volume; while the left renal echogenicity showed a weakly negative correlation with left renal breadth, height, and volume (Figures 3,4) both correlations were not statistically significant. Also, both right and left renal echogenicity showed a positive correlation with right and left renal lengths; and the correlation for the right being statistically significant. This implies that as right and left renal echogenicity increased there was an increase in renal length for the right and left kidneys with only the right being statistically significant. The right and left renal breadth, height, and volume did not show any significant statistically significant correlation with renal echogenicity.
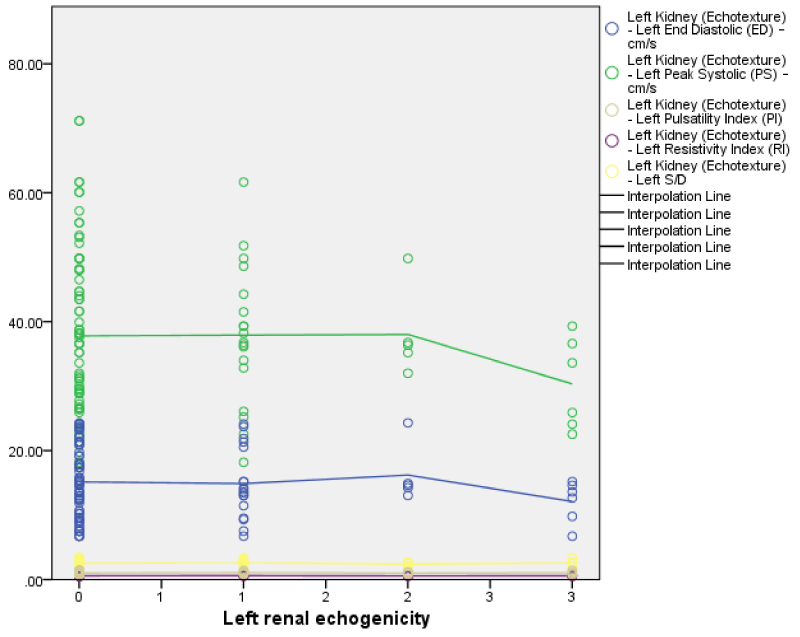
The right and left renal echogenicity showed a weakly negative correlation with right and left renal PSV, EDV, S/D, RI, and PI but only left RI was statistically significant (r = -0.18, p = 0.03) (Figure 16). This implies with an increase in the grade of renal echogenicity the RI, PSV, and EDV reduced while the S/D and PI increased. Comparing the left and right renal Doppler parameters with their renal echogenicity; there is a negative correlation between both renal Doppler parameters and its renal echogenicity with only left RI being statistically significant Figures 3-5.
Discussion
HIV/AIDS is a chronic morbid condition that has long reached epidemic proportions in all sub-Saharan African countries causing great economic losses due to an increase in mortality and morbidity. Recently, its mortality and morbidity rates have declined due to improvements in anti-retroviral therapy [10]. The direct cytopathic effect of the virus, the effect of opportunistic infections, and adverse reactions to a number of Antiretroviral Therapy (ART) impact negatively on the kidneys. This study set out to evaluate the association between HIV/AIDS and ultrasound (B mode and Doppler) parameters of the kidneys as well as correlate the measured parameters against prognostic markers like CD4 count, viral load, and serum creatinine [14].
A total of 200 subjects were recruited into this study which comprised 100 HIV seropositive subjects and 100 apparently healthy adults as controls. The age range of the study population was 19 to 77 years with a mean age of 41.4 ± 11.5 years for the HIV/AIDS patients and control subjects. This was similar to findings in a study on 340 HIV/AIDS subjects at a tertiary hospital in Lagos by Eze, et al. [31] who recorded a mean age of 42.7 ± 9.4years. The age group of 39 – 58 years accounted for 51 (51.0%) of the study population while the least affected age group was 57 – 78 years. The findings of a higher proportion of the older age group of 39 to 58 years as against the expected more sexually active age group of 19 to 38 years in this study may be due to the higher life expectancy of patients due to the use of ARV therapy. These findings are different from those of a previous study on renal sonographic parameters in HIV-Infected Subjects and their Relationship to CD4 Cell count conducted by Adeyekun, et al. [32] of 120 HIV/AIDS subjects in Benin City with a mean age of 36.84 ± 10.15years and modal age group distribution of 30 – 39 years as well as other studies done in Nigeria by Igbinedion, et al. [33], Ibinaiye, et al. [34], Ulu, et al. [35], Ekrikpo, et al. [36] who recorded younger mean ages and predominantly younger age distribution.
There were more females than males in this study with a male-to-female ratio of 1:2 which is similar to findings in studies involving 300 HIV/AIDS subjects in Benin city by Igbinedion, et al. [33] who recorded a male: female ratio of 1:2.4; 100 HIV/AIDS subjects in Maiduguri by Garko, et al. [20] and Ibinaiye, et al. [34] who both had a male: female ratio of 1:2 and 1,317 HIV/AIDS subjects at Uyo by Udeme, et al. [36] who recorded a male: female ratio of 1:1.6. However, there were a higher proportion of males in studies by Eze, et al. [31] at Lagos of 340 HIV/AIDS subjects with a male: female ratio of 1.2:1 and Emem, et al. [37] who studied 400 HIV/AIDS subject in two southern Nigeria cities and had a male: female of 1.1:1.
This finding of a higher female-to-male ratio in the prevalence of HIV infection in this study as a result of the routine practice of testing pregnant women for HIV during antenatal screening. Men are less likely to be offered such routine testing. Also, more women could be receiving ART, and have better adherence to medication which results in higher survival rates with HIV infection leading to higher HIV prevalence among women compared to men [33,38].
The study population was predominately Esan consisting of 74.0% and 60.0% for the HIV/AIDS and controls respectively. This finding may be a result of the study location and its environs being predominantly populated by individuals of Esan descent [39]. Most of the subjects were married with 65.0% and 75.0% for the HIV/AIDS and control subjects respectively. There were also more divorced and widowed subjects 12 (12.0%) and 2 (2.0%) respectively amongst the HIV cohort. This is probably due to a higher incidence of death of spouses from HIV/AIDS or divorce of discordant couples [40]. In a study by Garko, et al. [20], 80% of the HIV/AIDS subjects were married while in another study by Eze, et al. [31], only about 40.3% were married.
Among the different strata of occupation in this study group, a greater percentage of the study subjects were artisans and traders 73 (73.0%) and 48 (48.0%) for the study and control populations respectively. This finding is similar to those of Eze, et al. [31] and may indicate that HIV/AIDS is predominant among the self-employed (traders and artisans).
A comparison of the mean weight, height, and BMI showed lower values for the HIV/AIDS subject compared with control subjects. The weight loss and lower BMI may be attributable to the effect of HIV/AIDS disease progression and/or drug use on the individuals. Possible pathways for these include chronic diarrhea, dementia, depression, substance abuse, and wasting syndrome (accompanied by fever and diarrhea). Also, inadequate nutrition in the context of oral candidiasis resulting in difficulty in swallowing; anorexia, persistent nausea, and vomiting resulting from HIV treatment i.e. ART and macrolide antibiotics [41]. The use of protease inhibitors has been postulated to result in weight loss [41].
The HIV/AIDS subjects in this study had a higher mean CD4 cell count of 433.66 ± 339.325 cells/µL (range: 18 to 1,920 cells/µL) compared with controls. The males had a mean of 423.97 ± 387.917cells/µL while the females had a mean of 438.65 ± 314.479 cells/µL. Also, 23 (23.0%) had a CD4 count of less than 200 cells/µL which implies severe immunosuppression. These findings were at variance with reports by Ibinaiye, et al. [34] in a study of 100 HIV/AIDS subjects for the relationship of ultrasound echogenicity, serum creatinine level, and CD4 cell counts in patients with HIVAN at Maiduguri, most of the patients (96%) had a CD4 count of fewer than 200 cells/µL while only 4 patients (4%) had a CD4 of > 200 cells/µL. The range of CD4 counts in the study by Ibinaiye, et al. [34] was between 42 and 252 cells/µL. Eze, et al. [31] in a study of renal echogenicity as a predictor of HIVAN involving 340 HIV/AIDS subjects at Lagos, recorded a lower mean CD4 count of 153.1 ± 103.2 cells/µL and 121.9 ± 91.0 cells/µL for male and female respectively. Also, a study in Benin city involving 120 HIV/AIDS subjects by Adeyekun, et al. [32] had a lower mean CD4 count measuring 189.34 ± 142.18 cells/µL. The higher CD4 count of this study population signifies better immunity and is presumably due to the early commencement of ART. The WHO guideline on when patients are to commence ART was previously based on CD4 count less than 350 cells/µL. However, with the widespread availability of ART and the “Treat All” policy, the WHO in 2015 recommended the commencement of ART for all diagnosed HIV patients regardless of CD4 count [42]. Hence, more patients have a better chance of preventing CD4 cell depletion from overwhelming HIV infection [15,37]. Also, better and effective adherence to medication and improved counseling by health workers may be responsible [40].
The mean HIV viral load for the study population was 539,000.65 ± 183,133.86 copies/mL which was higher than values documented by Eze, et al. [31] with a mean viral load of 303,204.0 ± 24,812.9 copies/mL and 216,514.6 ± 18,823.0 copies/mL in male and female respectively. Also, only about 29 (29.0%) HIV/AIDS subjects in this study had unsuppressed viral load. This indicates that most patients in this study had better treatment outcomes. However, unsuppressed viral load may be due to the acute phase of RVD infection or seen in patients with poor response to ARV drugs.
The HIV/AIDS subjects had a mean serum creatinine of 98.35 ± 106.9mmol/dl which was significantly higher than that of the control subjects 74.80 ± 25.27mmol/dl. The lowest and highest values measured were 35.37mmol/dl and 831.15mmol/dl for HIV/AIDS subjects and 17.68mmol/dl and 150.31mmol/dl in the controls respectively. These values are lower compared with those of previous studies by Eze, et al. [31] who recorded mean serum creatinine levels of 218.4 ± 147.4 mmol/L and 222.0 ± 150.4 mmol/L for males and females respectively and also by Ibinaiye, et al. [34] which had an average serum creatinine level of 399 mmol/L; (range of 24mmol/L to 1,048mmol/L). These findings of less pronounced renal impairment in the index study may be also due to better management outcomes of the patients who were all on ARV, especially with regard to renal infection, fluid, and electrolyte balance.
The sonographic renal dimensions of the HIV/AIDS subjects in this study were higher than those of the control subjects. All renal parameters (length, breadth, height, and volumes) of both kidneys were significantly higher in the HIV/AIDS patients than in the controls. The findings of a larger kidney in HIV/AIDS subject compared with controls is similar to those in studies carried out by Garko, et al. [20] in Maiduguri with 100 HIV/AIDS subjects, Igbinedion, et al. [33] in Benin with 300 HIV/AIDS subjects, and Ibinaiye, et al. [34] in Maiduguri of 100 HIV/AIDS subjects. These findings are however at variance with those from the study by Obajimi, et al. [21] in Ibadan did not find any statistical difference in renal length and AP diameter between HIV-positive and HIV-negative control subjects. Garko, et al. [20] also postulates that renal enlargement in HIVAN is a result of insufficient time for global renal sclerosis and fibrosis, due to the rapid progression of renal disease; marked dilatation of the tubules with numerous microcysts, in contrast to the tubular collapse frequently seen in other forms of chronic renal injury; and interstitial edema [20,43,44].
In this study, 171 subjects consisting of all the control subjects 100 (100.0%) and 71 (71.0%) of HIV/AIDS subjects had normal grade 0 renal echogenicity and only about 29 (29%) had increased renal echogenicity with 18 (18.0%), 5 (5.0%) and 6 (6.0%) corresponding to grades 1, 2 and 3 respectively. There was a statistically significant difference in renal parenchymal echogenicity between the HIV/AIDS patients and controls respectively (p < 0.001). The proportion of subjects with increased echogenicity was lower in this study than in studies done by Adeyekun, et al. [32] which had 50 (41.7%) of the HIV/AIDS study population with increased echogenicity. Garko, et al. [20] had 96 (96%) HIV/AIDS patients with increased renal echogenicity and the majority 56 patients (56%) had grade III echogenicity, 4 patients (4%) had grade I echogenicity, and grade II echogenicity was seen in 36 patients (36%). Igbinedion, et al. [33] in a study, also found that the total number of patients with increased renal echogenicity decreased as the grade of cortical echogenicity increased which also corresponds with the findings in this study. Their findings were 73 (24.3%), 29 (9.7%), and 10 (2.2%) corresponding to Grades 1, 2, and 3 renal echogenicity. Also, Ulu, et al. [35], reported 77.7% of study subjects had increased renal parenchymal echogenicity. The findings of increased parenchymal echogenicity in the index study and other similar studies may be explained by kidney response to HIV infection which includes profound tubulo-interstitial scarring and atrophy [35,43].
In this study, the normal control subjects had a mean RI of 0.59 ± 0.05 with an RI of 0.59 ± 0.06 and 0.58 ± 0.05 for the right and left kidneys respectively. This was similar to mean values of normal controls in studies by Atalabi, et al. [45] whose study reported an RI of 0.56 in 68 healthy adults at Ibadan, Nejad, et al. [46] in a study involving 30 normal control at Hashemi-Nejad Hospital, Tehran reported an RI of 0.59, while Sari, et al. [47] reported an RI of 0.56 in 50 healthy controls at KTU Farabi Hospital, Turkey. However, Ishimura, et al. [48] in a Japanese study reported a higher RI of 0.66 for 37 control subjects and also reported a positive correlation between age and RI values. The higher mean age of the Japanese study which was 58.5 ± 12.5years compared to the mean ages of 48.87 ± 8.47years and 50.1 ± 13.7years recorded by Atalabi, et al. and Nejad, et al. could be responsible for the higher RI values for the Japanese study. Also, factors such as small sample size and possible racial variation may be responsible for the different RI values.
The HIV/AIDS subjects recorded a mean RI of 0.62 ± 0.05 with an RI of 0.63 ± 0.04 and 0.60 ± 0.05 for the right and left kidneys respectively. The RI value was statistically higher in HIV/AIDS subjects than in control subjects; p = < 0.001 for both kidneys. These findings are similar to differences seen in studies between normal controls and subjects with chronic nephropathies including diabetics and hypertensive nephropathies [45-50]. In studies comparing healthy control subjects and patients with diabetic nephropathy – Nejad, et al [46] reported mean RI of 0.73 for diabetic patients and 0.59 for controls (p = < 0.001), Ishimura, et al. [48] reported RI of 0.76 compared with 0.66 for controls (p = < 0.001), Sari, et al. [47] reported RI value of 0.69 compared with 0.56 for controls (p = < 0.001), Dawha, et al. [49] also reported RI of 0.72 compared with 0.63 for controls (p = < 0.001). Also, in studies comparing hypertensives with control subjects Atalabi, et al. [45] reported an RI of 0.595 in hypertensives compared to 0.56 for controls. RI has a strong positive correlation with chronic renal disease associated with active tubule-interstitial disease, vasculitis, and vasculopathy but poor correlation with chronic renal disease limited to the glomeruli such as glomerulonephritis [48,51]. In HIV infection, there is direct infection of epithelial cells of the nephron, including the glomerulus, the tubules, and the collecting duct with associated podocyte proliferation and tubular dilatation, atrophy and flattening of tubular epithelia cells, interstitial edema and inflammation and often accompany interstitial fibrosis which are all features of classic HIVAN [52,53]. Furthermore, renal parenchyma disease results in loss of diastolic flow indicating an increase in vascular resistance or increase in parenchymal pressure within the kidney resulting in an increased RI [26]. These tubulo-interstitial damages may be responsible for the increased vascular resistance resulting in the statistical difference of the renal Doppler RI between the HIV/AIDS and control subjects.
The mean renal Pulsatility Index (PI) for the control subjects measured 0.96 ± 0.20 with the right and left renal PI measuring 0.97 ± 0.19 and 0.94 ± 0.21 respectively. These findings were similar to those of Dawha, et al. [49] in a study of 90 apparently healthy controls at Ife recording a mean PI of 1.08 ± 0.20.
The mean renal Pulsatility Index (PI) in the index study was also significantly higher in the HIV/AIDS subjects mean of 1.06 ± 0.20 compared with 0.96 ± 0.20 in the control subjects, p < 0.001. These findings are similar to findings in chronic renal disease like diabetic nephropathy with Dawha, et al. [49] recording a mean PI of 1.36 ± 0.24 compared to controls with 1.08 ± 0.20, p < 0.0001. However, the differences in PI between subjects and controls were not as pronounced as those seen with RI. While RI is an indicator of resistance of an organ to perfusion and reflects downstream resistance in arteries; the PI is a measure of variability of the blood velocity in a vessel. Hence, the RI is a more sensitive parameter for renal vascular resistance than the PI due to a higher variability of PI compared to RI [54].
The mean renal Doppler parameters of PSV, EDV, and S/D were higher in the HIV/AIDS subjects compared with the control for both kidneys. This difference was statistically significant except for the left renal EDV. The PSV and EDV are more susceptible to variations in the angle of insonation and scanning factor and hence, are less reliable Doppler parameters than the RI and PI [55].
In this study increase in renal volumes of the right and left kidneys showed a weak negative correlation with CD4 count which was not statistically significant. This implies that with an increase in CD4 count, the renal volume decreases; although this change was not statistically significant. This finding is similar to findings in the study done by Di Fiori, et al. [56] on 152 kidneys of HIV/AIDS subjects in California who reported that subjects with smaller kidneys had higher CD4 counts and lived longer. This would imply that patients with larger kidneys had a more severe disease and hence poorer prognosis from HIV infection. Adeyekun, et al. [32] in a study of 120 HIV/AIDS patients at UBTH did not find any correlation between renal size and CD4 count even though about 74% of subjects had a CD4 count less than 200/mm3. This could imply that a very small portion of the subjects in the study by Adeyekun, et al. [32] had renal dysfunction; although the serum creatinine values were not assessed and this assertion could not be verified. However, in the index study about 13% of subjects had elevated serum creatinine > 1.35 mg/dL. Also, of all the renal dimensions (length, height, and breath) only the left renal height and length showed a statistically significant negative correlation with CD4.
The renal length, breadth, height, and volume did not show any statistically significant correlation with CD4, viral load, serum creatinine as well as with PSV, EDV, S/D, RI, and PI. Although the renal volume showed weak negative correlation with CD4, viral load, and serum creatinine (right: r = -0.08, -0.08, -0.12; p = 0.23, 0.22, 0.13 and left: r = -0.12, -0.02, -0.02; p = 0.11, 0.42, 0.42). This is similar to study findings by Igbinedion, et al [33] which did not find any statistically significant correlation between renal volume and CD4 count (p = 0.052). This finding may be due to better ART use which reduces the effects of HIV infection on the kidney hence the less pronounced effect with an increase in viral load [42].
In this study, both kidneys showed a statistically significant but weak negative correlation of renal echogenicity with CD4 count (r = -0.266; p = 0.004), and a statistically significant but weak positive correlation of renal echogenicity with viral load and serum creatinine (r = 0.325, 0.499; p = < 0.001, < 0.001). Also, there was no statistically significant correlation of renal echogenicity with renal length, breadth, height, and volume as well as PSV, EDV, S/D, RI, and PI.
These findings are similar to those from the study by Igbinedion, et al. [33] which found that a decrease in CD4 count was associated with a higher proportion of increased cortical echogenicity. It has been postulated that HIV infection results in glomerular changes, tubular dilation and rapidly rising creatinine levels and all these could be responsible for the increased echogenicity of the kidneys [6]. Eze, et al. [31] also noted a strong negative correlation between renal echogenicity and CD4 count (r was -0.610, -0.609, -0.514, and -0.621 for grades 0, 1, 2 and 3; p < 0.05).
This study recorded the three renal echogenicity grades as 36 (18%), 10 kidneys (5%), and 12 kidneys (6%) for grades 1, 2, and 3 with serum creatinine values of 80.56 ± 60.57mmol/L, 88.420 ± 26.53mmol/L and 369.89 ± 273.24 mmol/L respectively, these showed statistically significant positive correlation; r = p < 0.001. These findings were similar to a study by Garko, et al. [20] which found a statistically significant correlation between serum creatinine and the degree of renal echogenicity (r = 0.9). Patients with grade III renal cortical echogenicity (56%) had much higher values of raised creatinine levels (348-1048 mmol/l) as compared to lower values of raised creatinine (245 mmol/l) in those patients with grade I renal cortical echogenicity (4%).
This study did not find any statistically significant correlation between PSV, EDV, S/D, RI, and PI with CD4, viral load, or serum creatinine value. However, a weak negative correlation exists between the Doppler parameters and Serum creatinine. This implies that as the serum creatinine increases the Doppler parameters decreased slightly with changes in the left RI being statistically significant. These findings were at variance with studies on other chronic renal diseases like Diabetics which found a strong positive correlation between serum creatinine and RI [48,57-59]. This implies that RI increased with worsening renal function in diabetic nephropathy. Although the RI was significantly higher in HIV/AIDS patients than in controls, it, however, did not increase with increased serum creatinine value. Hence, the inference that RI does not serve as a good prognosticator of renal function in HIV/AIDS patients can be made.
Limitations of the study
- Renal volume measurement based on the ellipsoid formula may underestimate the renal volume.
- Extreme obesity made scanning very difficult for some subjects. Hence, the obese patients were scanned with an adjusted machine gain setting.
- The proportion of patients with immunosuppression and renal impairment was small in this study probably due to better therapeutic management of HIV/AIDS patients.
Conclusion
There was a significant increase in renal echogenicity, volume, and RI in HIV/AIDS subjects when compared with controls.
The renal echogenicity showed a weak but statistically significant correlation with CD4 count, viral load, and serum creatinine. The renal volume showed a weak correlation with CD4 count, viral load, and serum creatinine but this finding was not statistically significant. However, there was a poor correlation of RI with CD4 count, viral load, and serum creatinine. The serum creatinine correlates better with renal echogenicity than renal artery resistivity index and renal volume.
Recommendation
Findings from the study show that renal echogenicity correlates better with serum creatinine than renal artery resistivity index and renal volume. Therefore, routine assessment of the renal echogenicity in HIV/AIDS patients is recommended as it provides the best ultrasound prognostic marker for renal function.
Further studies incorporating large proportions of HIV/AIDS patients with and without renal dysfunction are recommended to validate this study’s findings and also to glean clinically relevant data on correlation with RI.
- Gallo RC. A reflection on HIV/AIDS research after 25 years. Retrovirology. 2006 Oct 20;3:72. doi: 10.1186/1742-4690-3-72. PMID: 17054781; PMCID: PMC1629027.
- Sepkowitz KA. AIDS--the first 20 years. N Engl J Med. 2001 Jun 7;344(23):1764-72. doi: 10.1056/NEJM200106073442306. PMID: 11396444.
- Campos P, Ortiz A, Soto K. HIV and kidney diseases: 35 years of history and consequences. Clin Kidney J. 2016 Dec;9(6):772-781. doi: 10.1093/ckj/sfw104. Epub 2016 Oct 25. PMID: 27994853; PMCID: PMC5162418.
- Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011 Sep;1(1):a006841. doi: 10.1101/cshperspect.a006841. PMID: 22229120; PMCID: PMC3234451.
- Symeonidou C, Standish R, Sahdev A, Katz RD, Morlese J, Malhotra A. Imaging and histopathologic features of HIV-related renal disease. Radiographics. 2008 Sep-Oct;28(5):1339-54. doi: 10.1148/rg.285075126. PMID: 18794311.
- Nathanson NM. Pathogenesis of AIDS: How does HIV casue AIDS? [Internet]. Pennsylvania: University of Pennsylvania, School of Medicine; 2012. http://www.pitt.edu/ ~super7/19011-20001/19601
- Galloway NL, Doitsh G, Monroe KM, Yang Z, Muñoz-Arias I, Levy DN, Greene WC. Cell-to-Cell Transmission of HIV-1 Is Required to Trigger Pyroptotic Death of Lymphoid-Tissue-Derived CD4 T Cells. Cell Rep. 2015 Sep 8;12(10):1555-1563. doi: 10.1016/j.celrep.2015.08.011. Epub 2015 Aug 28. PMID: 26321639; PMCID: PMC4565731.
- Mzingwane ML, Tiemessen CT. Mechanisms of HIV persistence in HIV reservoirs. Rev Med Virol. 2017 Mar;27(2). doi: 10.1002/rmv.1924. Epub 2017 Jan 27. PMID: 28128885.
- Miller FH, Parikh S, Gore RM, Nemcek AA Jr, Fitzgerald SW, Vogelzang RL. Renal manifestations of AIDS. Radiographics. 1993 May;13(3):587-96. doi: 10.1148/radiographics.13.3.8316666. PMID: 8316666.
- Wyatt CM. Kidney Disease and HIV Infection. Top Antivir Med. 2017 Feb/Mar;25(1):13-16. PMID: 28402929; PMCID: PMC5677039.
- da Silva DR, Gluz IC, Kurz J, Thomé GG, Zancan R, Bringhenti RN, Schaefer PG, Dos Santos M, Barros EJ, Veronese FV. Multiple facets of HIV-associated renal disease. Braz J Med Biol Res. 2016;49(4):e5176. doi: 10.1590/1414-431X20165176. Epub 2016 Mar 18. PMID: 27007656; PMCID: PMC4819412.
- Chongwe G, Michelo C. Renal Dysfunction among HIV-infected Patients on Tenofovir-Based Antiretroviral Therapy at Ronald Ross Hospital in Zambia. Omi Int Res Artic J AIDS Clin Res. 2017;8:651.
- Wyatt CM, Klotman PE, D'Agati VD. HIV-associated nephropathy: clinical presentation, pathology, and epidemiology in the era of antiretroviral therapy. Semin Nephrol. 2008 Nov;28(6):513-22. doi: 10.1016/j.semnephrol.2008.08.005. PMID: 19013322; PMCID: PMC2656916.
- Mellors JW, Muñoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR Jr. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997 Jun 15;126(12):946-54. doi: 10.7326/0003-4819-126-12-199706150-00003. PMID: 9182471.
- Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O'Shaughnessy MV, Montaner JS. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001 Nov 28;286(20):2568-77. doi: 10.1001/jama.286.20.2568. PMID: 11722271.
- Bennett J, Dolin R, Blaser MJ. Mandell, Dougla, and Bennett’s Principles and Practice of Infectious Disease. 9th ed. 2015;41–55.
- World Health Organisation. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. 2007;1-48.
- Fredrick F, Francis JM, Ruggajo PJ, Maro EE. Renal abnormalities among HIV infected children at Muhimbili National Hospital (MNH)-Dar es Salaam, Tanzania. BMC Nephrol. 2016 Mar 21;17:30. doi: 10.1186/s12882-016-0242-6. PMID: 27000018; PMCID: PMC4800772.
- Post FA, Holt SG. Recent developments in HIV and the kidney. Curr Opin Infect Dis. 2009 Feb;22(1):43-8. doi: 10.1097/QCO.0b013e328320ffec. PMID: 19106702.
- Garko SS, Ibinaiye P, Abba S, Ahmed A, SS T, Okere P. The utilization of diagnostic ultrasound in the evaluation of the kidneys in HIV-associated nephropathy. West Afr J Radiol. 2015;22:20-26.
- Obajimi MO, Atalabi MO, Ogbole GI, Adeniji-Sofoluwe AT, Agunloye AM, Adekanmi AJ, Osuagwu YU, Olarinoye SA, Olusola-Bello MA, Ogunseyinde AO, Aken'Ova YA, Adewole IF. Abdominal ultrasonography in HIV/AIDS patients in southwestern Nigeria. BMC Med Imaging. 2008 Feb 29;8:5. doi: 10.1186/1471-2342-8-5. PMID: 18312644; PMCID: PMC2275264.
- Cochran WG. Sampling techniques. 3rd ed. New York: John Wilery & Sons. 1977. 1-428.
- Araoye M. Research Methodology with Statistics for Health and Social Sciences. Illorin: Nathadex Publishers. 2004. 116–125.
- NACA. HIV and AIDS in Nigeria: Nigeria updated August 2017. Abuja/Geneva. 2016; 1–10. http://naca.gov.ng/naca-annual-report-2016
- Blackburn H, Jacobs D Jr. Commentary: Origins and evolution of body mass index (BMI): continuing saga. Int J Epidemiol. 2014 Jun;43(3):665-9. doi: 10.1093/ije/dyu061. Epub 2014 Apr 1. PMID: 24691955.
- Federal Ministry of Health. National Guidelines for HIV Prevention Treatment and Care. Abuja. 2016.
- Rosenfield AT, Siegel NJ. Renal parenchymal disease: histopathologic-sonographic correlation. AJR Am J Roentgenol. 1981 Oct;137(4):793-8. doi: 10.2214/ajr.137.4.793. PMID: 6974977.
- Sharma K, Caroli A, Quach LV, Petzold K, Bozzetto M, Serra AL, Remuzzi G, Remuzzi A. Kidney volume measurement methods for clinical studies on autosomal dominant polycystic kidney disease. PLoS One. 2017 May 30;12(5):e0178488. doi: 10.1371/journal.pone.0178488. PMID: 28558028; PMCID: PMC5448775.
- Myron PA, Paul AL. Clinical doppler ultrasound. 3rd ed. Edinburgh: Churchill Livingstone Elsevier. 2014; 193–213.
- IBM Corporation. IBM How to cite IBM SPSS Statistics or earlier versions of SPSS. IBM Support. 2019;1. https://www-01.ibm.com/support/docview.wss?uid=swg21476197
- Eze CU, Adeyomoye AAO. Renal Echogenicity as a Predictor of Human Immunodeficiency Virus–Associated Nephropathy: A Cross-Sectional Study at a Tertiary Hospital in Lagos, Southwest Nigeria. J Diagnostic Med Sonogr. 2018;34:261-272.
- Adeyekun AA, Unuigbe EI, Onunu AN, Azubike CO. Renal sonographic parameters in human immunodeficiency virus - infected subjects and relationship to CD4 cell count. Saudi J Kidney Dis Transpl. 2011 Nov;22(6):1164-8. PMID: 22089775.
- Igbinedion B, Marchie T, Ogbeide E. Trans-abdominal ultrasonic findings correlated with CD4+ counts in adult HIV infected patients in Benin, Nigeria. SA J Radiol. 2009;13:34-40.
- Ibinaiye P, Tahir N, Tanimu S, Ahmed A, Garko S. Relationship of ultrasound renal echogenicity, serum creatinine level and CD4 cell counts in patients with human immunodeficiency virus-associated nephropathy.Sub-Saharan African J Med. 2014;1:191-197.
- Ulu UO, Agbaji O, Agwu KK. Sonographic characterization of renal pathologies in HIV/AIDS in Plateau State, Nigeria. Niger J Med. 2012 Apr-Jun;21(2):160-4. PMID: 23311183.
- Ekrikpo UE, Kengne AP, Akpan EE, Effa EE, Bello AK, Ekott JU, George C, Salako BL, Okpechi IG. Prevalence and correlates of chronic kidney disease (CKD) among ART-naive HIV patients in the Niger-Delta region of Nigeria. Medicine (Baltimore). 2018 Apr;97(16):e0380. doi: 10.1097/MD.0000000000010380. PMID: 29668591; PMCID: PMC5916672.
- Emem CP, Arogundade F, Sanusi A, Adelusola K, Wokoma F, Akinsola A. Renal disease in HIV-seropositive patients in Nigeria: an assessment of prevalence, clinical features and risk factors. Nephrol Dial Transplant. 2008 Feb;23(2):741-6. doi: 10.1093/ndt/gfm836. Epub 2007 Dec 8. PMID: 18065807.
- Hegdahl HK, Fylkesnes KM, Sandøy IF. Sex Differences in HIV Prevalence Persist over Time: Evidence from 18 Countries in Sub-Saharan Africa. PLoS One. 2016 Feb 3;11(2):e0148502. doi: 10.1371/journal.pone.0148502. PMID: 26841112; PMCID: PMC4739589.
- Omorodion FI. The socio-cultural context of health behaviour among Esan communities, Edo State, Nigeria. Health Transit Rev. 1993 Oct;3(2):125-36. PMID: 10146569.
- Kharsany AB, Karim QA. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016 Apr 8;10:34-48. doi: 10.2174/1874613601610010034. PMID: 27347270; PMCID: PMC4893541.
- Williams B, Waters D, Parker K. Evaluation and treatment of weight loss in adults with HIV disease. Am Fam Physician. 1999 Sep 1;60(3):843-54, 857-60. PMID: 10498111.
- Johnson SC. Antiretroviral Therapy for HIV Infection: When to Initiate Therapy, Which Regimen to Use, and How to Monitor Patients on Therapy. Top Antivir Med. 2015;23:161.
- Medapalli RK, He JC, Klotman PE. HIV-associated nephropathy: pathogenesis. Curr Opin Nephrol Hypertens. 2011 May;20(3):306-11. doi: 10.1097/MNH.0b013e328345359a. PMID: 21358326; PMCID: PMC3153858.
- Glassock RJ, Cohen AH, Danovitch G, Parsa KP. Human immunodeficiency virus (HIV) infection and the kidney. Ann Intern Med. 1990 Jan 1;112(1):35-49. doi: 10.7326/0003-4819-112-1-35. Erratum in: Ann Intern Med 1990 Mar 15;112(6):476. PMID: 2403474.
- Atalabi OM, Yusuf BP. Comparative Ultrasound Evaluation of Renal Resistive Index in Hypertensive and Normotensive Adults in Ibadan , SouthWestern , Nigeria. Trop J Nephrol. 2012;7:19-26.
- Nejad NM, Jafari B, Alipour P. Arterial resistive index (RI) in type II diabetic nephropathy stages and healthy controls. Iran J Radiol. 2009;6:29-32.
- Sari A, Dinc H, Zibandeh A, Telatar M, Gümele HR. Value of resistive index in patients with clinical diabetic nephropathy. Invest Radiol. 1999 Nov;34(11):718-21. doi: 10.1097/00004424-199911000-00008. PMID: 10548384.
- Ishimura E, Nishizawa Y, Kawagishi T, Okuno Y, Kogawa K, Fukumoto S, Maekawa K, Hosoi M, Inaba M, Emoto M, Morii H. Intrarenal hemodynamic abnormalities in diabetic nephropathy measured by duplex Doppler sonography. Kidney Int. 1997 Jun;51(6):1920-7. doi: 10.1038/ki.1997.261. PMID: 9186883.
- Dawha S, Oo A, Bo I, Rt I, Fa A. An assessment of factors influencing resistivity and pulsatility indices in diabetes mellitus. Trop J Nephrol. 2014;9:15-22.
- Yusuf BP, Atalabi OM, Yusuf BP. Renal Resistive Index in Normal Adults. in-Ibadan, Southwestern Nigeria: A Preliminary Report. West African J Ultrasound. 2010;11:12-6.
- Mocroft A, Lundgren JD, Ross M, Law M, Reiss P, Kirk O, Smith C, Wentworth D, Neuhaus J, Fux CA, Moranne O, Morlat P, Johnson MA, Ryom L; D:A:D study group; Royal Free Hospital Clinic Cohort; INSIGHT study group; SMART study group; ESPRIT study group. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med. 2015 Mar 31;12(3):e1001809. doi: 10.1371/journal.pmed.1001809. PMID: 25826420; PMCID: PMC4380415.
- Naicker S, Rahmanian S, Kopp JB. HIV and chronic kidney disease. Clin Nephrol. 2015;83(7 Suppl 1):32-8. doi: 10.5414/cnp83s032. PMID: 25725239; PMCID: PMC4536633.
- Fine DM, Perazella MA, Lucas GM, Atta MG. Renal disease in patients with HIV infection: epidemiology, pathogenesis and management. Drugs. 2008;68(7):963-80. doi: 10.2165/00003495-200868070-00006. PMID: 18457462.
- Spatola L, Andrulli S. Doppler ultrasound in kidney diseases: a key parameter in clinical long-term follow-up. J Ultrasound. 2016 Apr 16;19(4):243-250. doi: 10.1007/s40477-016-0201-x. PMID: 27965714; PMCID: PMC5126008.
- Petersen LJ, Petersen JR, Talleruphuus U, Ladefoged SD, Mehlsen J, Jensen HA. The pulsatility index and the resistive index in renal arteries. Associations with long-term progression in chronic renal failure. Nephrol Dial Transplant. 1997 Jul;12(7):1376-80. doi: 10.1093/ndt/12.7.1376. PMID: 9249772.
- Di Fiori JL, Rodrigue D, Kaptein EM, Ralls PW. Diagnostic sonography of HIV-associated nephropathy: new observations and clinical correlation. AJR Am J Roentgenol. 1998 Sep;171(3):713-6. doi: 10.2214/ajr.171.3.9725302. PMID: 9725302.
- Platt JF, Rubin JM, Ellis JH. Diabetic nephropathy: evaluation with renal duplex Doppler US. Radiology. 1994 Feb;190(2):343-6. doi: 10.1148/radiology.190.2.8284379. PMID: 8284379.
- MNBJPA. Urogenical Imaging: Arterial Resistive Index (RI) in Type II Diabetic Nephropathy Stages and Healthy Controls. Iran J Radiol. 2009;6:29-32.
- Brkljacić B, Mrzljak V, Drinković I, Soldo D, Sabljar-Matovinović M, Hebrang A. Renal vascular resistance in diabetic nephropathy: duplex Doppler US evaluation. Radiology. 1994 Aug;192(2):549-54. doi: 10.1148/radiology.192.2.8029430. PMID: 8029430.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
