Journal of Vaccines and Immunology
Characterization of enterotoxigenic Escherichia coli heat-labile toxin (LT) double mutant LTA72R/R192G as a Nontoxic and Effective Mucosal Adjuvant
Li Ben-Qiang1, Tao Jie1, Cheng Jing-Hua1, Shi Ying1 and Liu Hui-li2*
2Institute of Animal Science and Veterinary Medicine, Shanghai Academy of Agricμltural Sciences, 201106, Shanghai, China
Cite this as
Ben-Qiang L, Jie T, Jing-Hua C, Ying S, Hui-li L (2022) Characterization of enterotoxigenic Escherichia coli heat-labile toxin (LT) double mutant LTA72R/R192G as a Nontoxic and Effective Mucosal Adjuvant. J Vaccines Immunol 8(1): 033-039. DOI: 10.17352/jvi.000053Copyright
© 2022 Ben-Qiang L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The heat-labile enterotoxins of Escherichia coli (LT) protein were reported to be an ideal mucosal adjuvant for nasal or oral delivery with antigen. Because of its toxicity, the application of native LT protein was focused on the purification of subunit B (LTB) or reconstructing non-toxic LT mutants, such as LTK63, LTR72, LTG192, or LTK63/R72. In this study, we mutated nucleotides coding the rd63,sup>rd and nd72nd residues, as well as the nd72nd, and 192th amino acids, to explore whether the double mutant LTA72R/R192G had a good adjuvanticity the same as the single mutant LTS63K or LTA72R, which were known as the ideal mucosal adjuvants. LTA72R/R192G was harmless to the mice because of the reduction of the ADP- ribosylation activity and toxicity. Besides, it could significantly enhance the mucosal immune response against the NCDV or CSFV antigens. In conclusion, the nontoxicity LTA72R/R192G may potentially serve as an effective adjuvant for mucosal immunization.
Introduction
Most pathogens invade the body through the mucosa [1]. Mucosal immunization is the most effective way for protecting against pathogens entering via the mucosal surface, which can directly target IgA antibody responses in the respiratory and digestive system, providing effective protection against airborne pathogens[2-4]. Furthermore, it has been confirmed that the mucosal IgA response exhibits a cross-protective immunity against antigenically distinct virus strainsμg [4,5].
Researchers take interested in developing effective mucosal adjuvants because the non-effective of the administered vaccines on stimμlating mucosal immune responses s are not μl [1,3,6,7]. Embedding of an adjuvant can improve the immunogenicity of the mucosal-delivered antigens especially for those that are weakly antigenic, thus enhancing the efficacy of vaccine products. Heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) and cholera toxin (CT) of Vibrio cholerae are the most studied mucosal adjuvants to date [8,9]. However, they cannot be used as mucosal adjuvants for human vaccines because of their potential toxin. Instead, low- or non-toxic LT or CT mutants are promising mucosal adjuvants [10-12].
The studies seem to indicate that LTS63K (Serine at the 63 residues replaced with Lysine), LTA72R, and LTR192G might be the most promising adjuvants. LTS63K and LTA72R were derived from a single site mutation at the ADP-ribosyltransferase active site LTA1 domain. LTR192G mutant has a single amino acid substitution at the trypsin-sensitive loop of the LTA subunit, which renders LT resistance to trypsin-mediated cleavage. It was reported that LTA72R had stronger mucosal adjuvant activity than LTS63K [13]. Thoμgh the mutant LTR192G showed good adjuvanticity, however, the enterotoxicity residues limited its application [14].
Compared with the single mutant, the double mutants at the key toxic active sites and/or the trypsin sensitivity sites may be more stable and less toxic. To screen an effective mucosal adjuvant candidate, a panel of single and double mutants of porcine-type LT toxin was constructed and comparative studies were carried out to explore the possibility of being a mucosal adjuvant. We selected the LaSota strain of New Castle Disease virus vaccine (NCDV), and Classical Swine Fever Virus (CSFV) vaccine as the co-antigens in the mice model to evaluate the mucosal adjuvanticity.μl.
Material and method
Vaccine strain
The commercially available LaSota vaccine strain against NCDV was produced by SongJiang Bio-product Factory, in Shanghai, China. CSFV was obtained from Qilu Animal HealthCare Co., SD, China.
All the commercial vaccines were produced according to Good Manufacturing Practice guidance and were used in the experiments for evaluating mucosal adjuvanticity.
LT gene amplification and mutation
The LT gene (eltAB) amplified from a porcine original enterotoxigenic E. coli K88ac strain (Veterinary Bacteria Stock Preservation Center, Chinese Institute of Veterinary Drug Control) and cloned into vector pET30a (+) (Novagen Merck Biosciences, German), named as pET30a-LT. Using pET30a-LT as the template, site-directed mutants were constructed. Upper stream primer 5’-agccatgggcaatggcgacagattatacc-3’ (forward) and downstream primer 5’-acgtcgacgtttttcatactgattgccgc-3’ (reverse) were used to amplify the cloned LT genes. Primers upper mut1 5’-cggatatgtttccactaaacttagtttgagaagtgctc-3’ (forward) and lower mut1 5’-gagcacttctcaaactaagtttagtggaaacatatccg-3’ (reverse) were used to mutate the nucleotides coding the 63rd amino acid (underlined nucleotides indicated the S→K substitution). SOEing PCR was carried out for single-site mutant gene amplification. Similarly, upper primers mut2 5’-agaagtgctcacttacgtggacagtctatattatcagg-3’ and lower mut2: 5’-cctgataatatagactgtccacgtaagtgagcacttctc-3’ were designed to introduce the A→R substitution at nucleotides coding the 72nd amino acid, and upper primers mut3: 5’-ggttgtgaaattcatcaggaacaatcacaggtgatacttg-3’ and downstream mut3: 5’-caagtatcacctgtgattgttcctgatgaatttccacaacc-3’ was used for the R→G mutation at the 192th amino acid of LT. All the primers were synthesized by Invitrogen (Carlsbad, CA, USA). PCR products were digested with NcoI and SalI restriction enzymes (New England Biolabs Co., MA, USA), and then cloned into vector pET30a (+). Positive colonies were screened by PCR and restriction enzyme digestion and verified with DNA sequencing.
The double-mutant LTS63K/A72R, LTS63K/R192G, and LTA72R/R192G genes were amplified using pET30a-LTR72 and pET30a-LTK63 as the templates.
Expression and purification of LT mutant proteins
The recombinant plasmids were transformed into the E. coli BL21 (DE3) (Stratagene, La Jolla, CA) competent cells. Added 1 mM isopropyl-1-thio-D-galactopyranoside (IPTG) (Gold Bio-technology, St. Louis, MO USA) at an optical density 600 of 0.5-0.6, inducible expression was carried out at 37 for 4 hours. Finally, the expressed bacteria solutions were harvested followed by centrifμgating μlμlμg at 4000X rpm (JA21 rotor,Beckman, Indianapolis, IN, USA) for 30 min.
Pellets were suspended in suspension buffer (20mM Tris-HCl, 100nM NaCl; pH 8.0) and then sonicated (Sonics’ VCX750 sonicator, Newtown, CT, USA) for 10 min. After centrifugation, pellets were suspended in urea-NTA buffer (20 mM Tris-HCl, 0.5 M NaCl, 10℅Glycerol, 8 M Urea; pH 7.9). Then, the recombinant proteins were purified using a Ni-NTA resin column (Invitrogen, Carlsbad, CA, USA) and FPLC (Amersham, Piscataway, NJ). Urea-NTA buffer containing 500 mM imidazole (Sigma, St. Louis, MO, USA) was used to elute the his-tagged recombinant proteins. Finally, 8M, 6M, 4, 2M, and 0M urea were used for dialysis and renaturation in turn.
The purified proteins were detected by SDS-PAGE and Western-blot using both the anti-His-tag (Tiangen Biotech Co., Beijing, China) and anti-CTB monoclonal antibodies (Calbiochem Co., LaJolla, CA, USA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Calbiochem Co., LaJolla, CA, USA) was used as the secondary antibody. Concentrations of the extracted proteins were assessed by Bicinchoninic Acid (BCA) (Pierce, Rockford, IL, USA) method. All proteins were stored at –80 ºC.
ADP-ribosyltransferase activity test (DEABAG assay)
The ADP-ribosyltransferase activities of the native LT and LT mutants were determined using p-diethylamino-benzylidineamino-guanidine (DEA-BAG) as substrate [15]. For each assay, 750 ng of each mutant LT protein was mixed with 5 μg trypsin (Sino-American Biotechnology Co., Luoyang, China) in 20 µl reaction buffer (50 mM Tris, 20 mM NaCl, 1 mM EDTA, 3 mM NaN3, 200 mM K2HPO4; pH 7.5) and incubated at 370C for 1 h. Trypsinization was stopped by adding 10 μg soybean trypsin inhibitor (Xitang Biological Sci-tech Co., Shanghai, China), followed by the addition of 200 μl of assay buffer (20mm DTT, 0.1 mg/ml BSA, 0.1% Triton X-100, and 2 mM DEA-BAG in PBS). ADP-ribosylation reaction was started by adding 25 μl of 100 mM NAD. After incubation for 2 h at 300C, unreacted DEABAG was removed by adsorption to a DOWEX resin (1ml 200 mM phosphate buffer, pH7.5, with 0.3g DOWEX resin) and after centrifugation, the supernatant was assayed for absorbance at 355 nm with DU-7 spectrophotometer (Beckman Instruments, Inc., Palo Alto, CA). The amount of product was calculated using standard curves generated with native LT protein [15,16].
In vivo enterotoxicity assay (orally and intranasally)
Forty-eight 6-week-old female Kunming mice (Experimental Animal Center, Fudan University, Shanghai, China) were randomly divided into eight groups. The mice were administered intragastrically with 100μg LT or mLT mutant protein and the control was administered with PBS. Mice were sacrificed after 6h, and the gut and body carcass were weighted. And the gut/carcass (G/C) ratios were further calculated and analyzed (Rachel C, et al. 2002).
Two groups of 4-week-old Kunming mice were administered intranasal either with 10 μg LTA72R/R192G protein or LT toxins for toxicity determination. The control was administrated with PBS. The mice have inoculated twice the interval of 14 days and two mice were sacrificed at 4h, 8h, 12h, 24h, and 48h after the second inoculation. The paraffin section was made from excised nasal mucosa, trachea, lung, and liver for microscopic examination.
The mouse study fully complied with the Guide for Care and Use of Laboratory Animals (8th edition, 2011).
Mouse immunization with Newcastle disease virus vaccine
6-week-old female BALB/c mice (Shanghai Laboratory Animal Center; Shanghai, China) were divided into 7 groups with ten of each group. Mice were immunized intranasally (i.n.) with either a dose of NCDV alone or a dose of NCDV combined with 4 μg of LT (or LT mutant protein). Mice were immunized three times over the interval of 12 and 14 days. Serum and nasal samples were collected one day before each inoculation. The nasal samples were collected by flushing the nasal cavities with 500 μl PBS containing 0.1% BSA. The samples were used for IgG and Ig A detection.
Hemagglutination inhibition (HI) assay
To perform the HI assay, pretreated sera were serially 2-fold diluted, mixed with the diluted virus 1:1, and incubated at room temperature for 60 min. Next, 50 μl of 1% chicken erythrocyte suspension was added, followed by incubation at room temperature for 60 min. Assay plates were tilted to read, and the titer was reported as the reciprocal of the highest serum dilution in which agglutination was completely inhibited (Bibby, et al. 2022).
Mouse immunization with classic Swine fever virus vaccine
Forty-eight 6-week-old female Kunming mice (Shanghai Laboratory Animal Center; Shanghai, China) were divided into five groups with eight of each. Group I was orally immunized with two doses CSFV vaccine, and group II was orally immunized with the CSFV vaccine with LTA72R/R192G protein. Group III and Group IV were intranasally immunized with the same dose of CSFV vaccine with or without LTA72R/R192G, respectively. Group V was intramuscularly immunized with two doses of CSFV vaccine with LTA72R/R192G protein.
The mice were immunized three times with the interval of 14 days respectively. The sera and nasal lavage fluid (with 500μl PBS+0.1% BSA) were collected on 0, 9, 17, 24, 32, 39 dpi. Serum samples and the nasal lavage fluid were collected for IgG and IgA antibody detection.
The mice were immunized three times with the interval of 14 days respectively. The sera and nasal lavage fluid (with 500μl PBS+0.1% BSA) were collected on 0, 9, 17, 24, 32, 39 dpi. Serum samples and the nasal lavage fluid were collected for IgG and IgA antibody detection.
c96-well high absorbance plates (Corning Costar, Lowell, USA) were coated with 100 μl of NCDV or CSFV at 4 °C overnight. After the plates were washed three times with PBS containing 0.05% Tween-20, a blocking buffer containing 5% BSA was added to the plate and incubated at 37 °C for 1 h. Then, samples (1:20 in dilution of serum and 1:40 dilution of nasal samples) were added to the plate and incubated at 37 °C for 1 h. Goat anti-mouse HRP-IgG or HRP-IgA (Calbiochem Co., LaJolla, CA, USA) were used as the secondary antibodies to detect a response from serum samples, and goat anti-mouse IgA was used to detect anti-NCDV antibody response from the nasal washing samples. OD450 values were measured after incubation with O-phenylenediamine substrate (Sangon Biotech Co., Shanghai, China) for 15 min at room temperature.
Data analysis
The OD values of the ELISA assay were calculated in geometric mean ± S.D. unless stated otherwise. Differences between treatment groups were calculated using Student’s t-test at a confidence level of 95%. A p -value of less than 0.05 (p < 0.05) was considered a statistically significant difference.
Results
LT mutants showed reduced ADP-ribosyltransferase activities
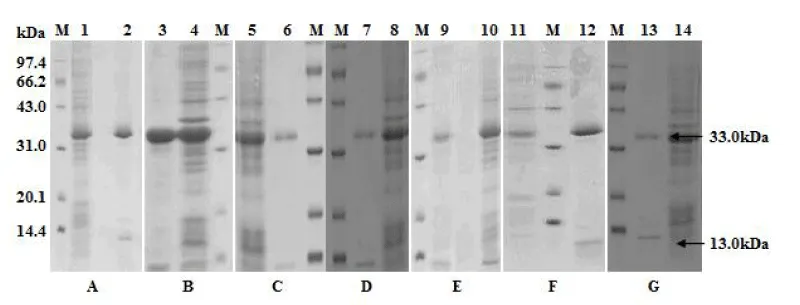
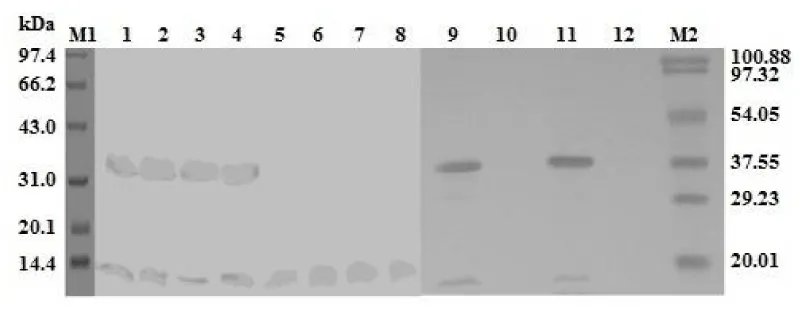
Molecules of 33 kDa and 13 kDa, equivalent to the his-tagged LTA subunit and LTB subunit, were detected from each LT mutant (Figure 1). These two subunits were verified by anti-His monoclonal antibody and anti-CTB polyclonal antibodies (Figure 2).
Using NAD+ start the reaction with DEABAG substrates. OD355 was used for the calculation of suspended concentration according to the standard curve for absorbance of DEABAG. The result indicates that all the mutants’ ADP-ribosylation activities were reduced compared with wild LT proteins. LTA72R/R192G is lower than LTA72R (Table 1).
LTA72R/R192G showed toxicity reduced
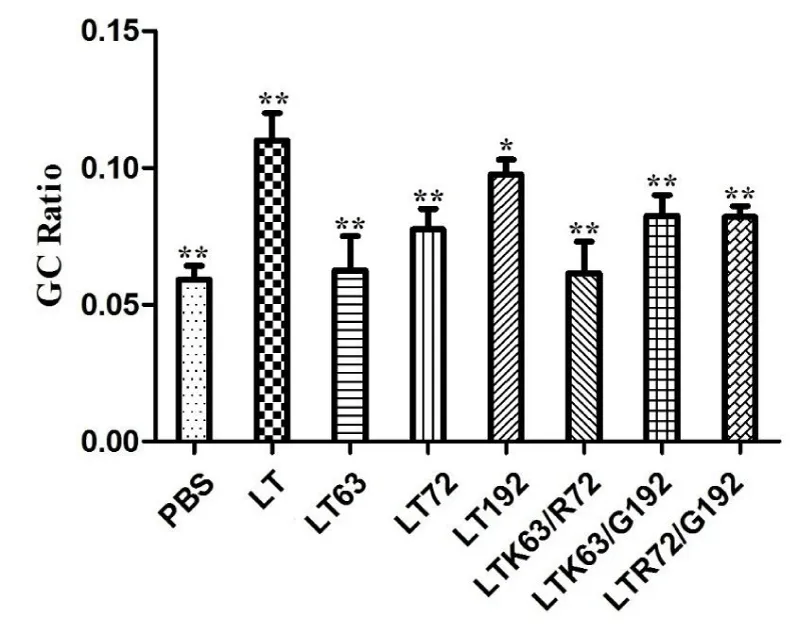
All mutant proteins showed reduced toxicity compared to native LT, indicated by mouse G/C ratios from mouse enterotoxicity assay. Among all tested mutants, LTA72R/R192G and LTS63K/A72R showed low toxicity based on the mouse toxicity assay (p > 0.05) (Figure 3).
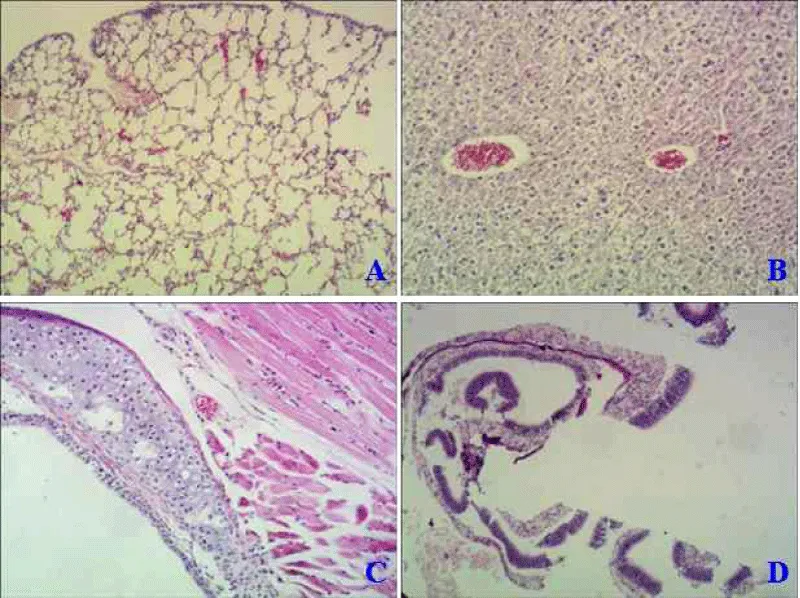
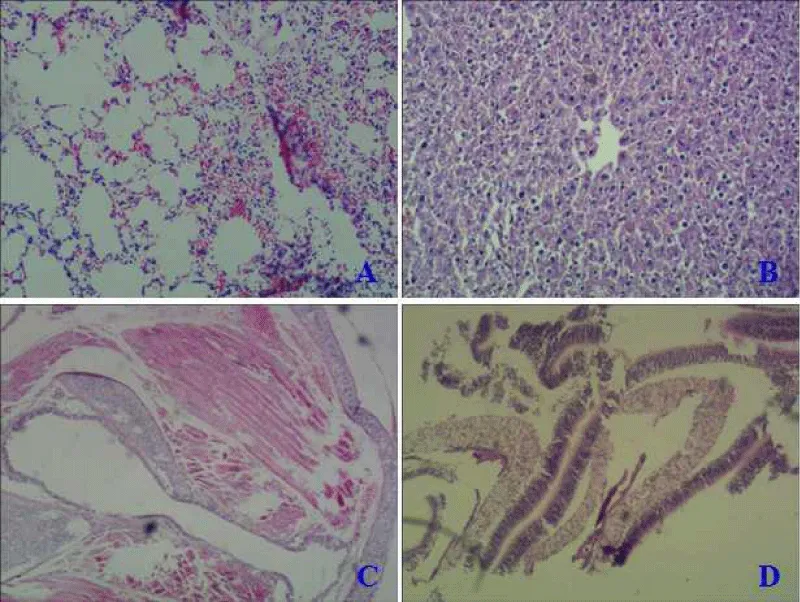
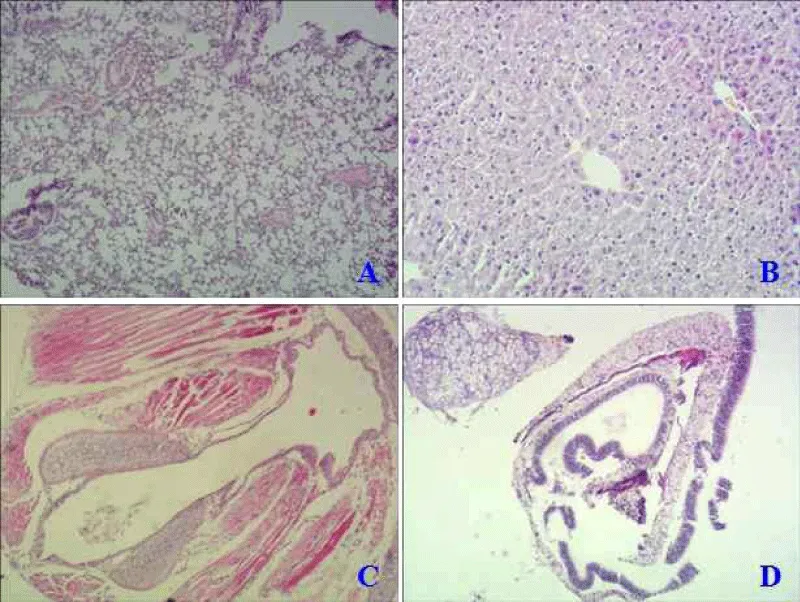
The paraffin section was made from excised nasal mucosa, trachea, lung, and liver of mice in an intranasal way to detect mutant protein’s toxicity. Compared with the normal mice (Figure 4A), native LT protein could harm the lung with obvious inflammatory lesions, thickening of alveolar walls, and significant inflammatory cellμlar infiltration (Figure 5A). While LTA72R/R192G showed no toxicity to immunized mice in all tissues (Figure 6 -Dμl).
LT mutant proteins enhanced mucosal immunity to the NCDV vaccine
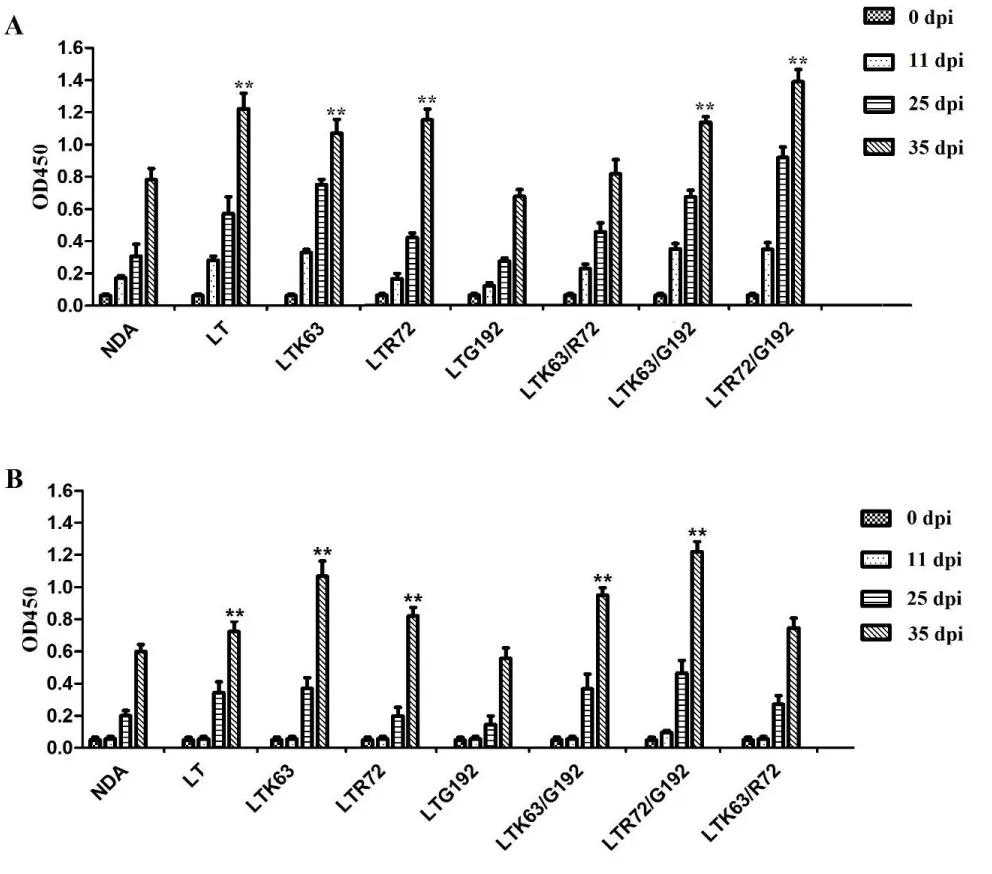
Serum anti-NCDV IgG antibodies in the mice immunized with NCDV with LTA72R/R192G or LTS63K/R192G proteins were significantly higher than that of the mice immunized with NCDV (p < 0.05; Figure 7A). Anti-NCDV mucosal IgA antibody response from the nasal washes of mice immunized with of the vaccine adjuvanted with LTA72R/R192G or LTS63K/R192G protein was significantly higher than that of the mice immunized with the NCDV vaccine (p < 0.05; Figure 7B). No anti-NCDV IgG or IgA antibodies were detected in the control mice.
Furthermore, we developed HI titers in serum and showed that anti-NCDV HI titers in the serum of the immunized mice adjuvanted with LT mutant proteins were higher than that of vaccinated with NCDV which was 26. HI, titers of mice immunized with the NCDV vaccine adjuvanted with LTA72R/R192G protein were the highest among the groups and were 210 .
LT mutant proteins showed adjuvanticity with the CSFV vaccine
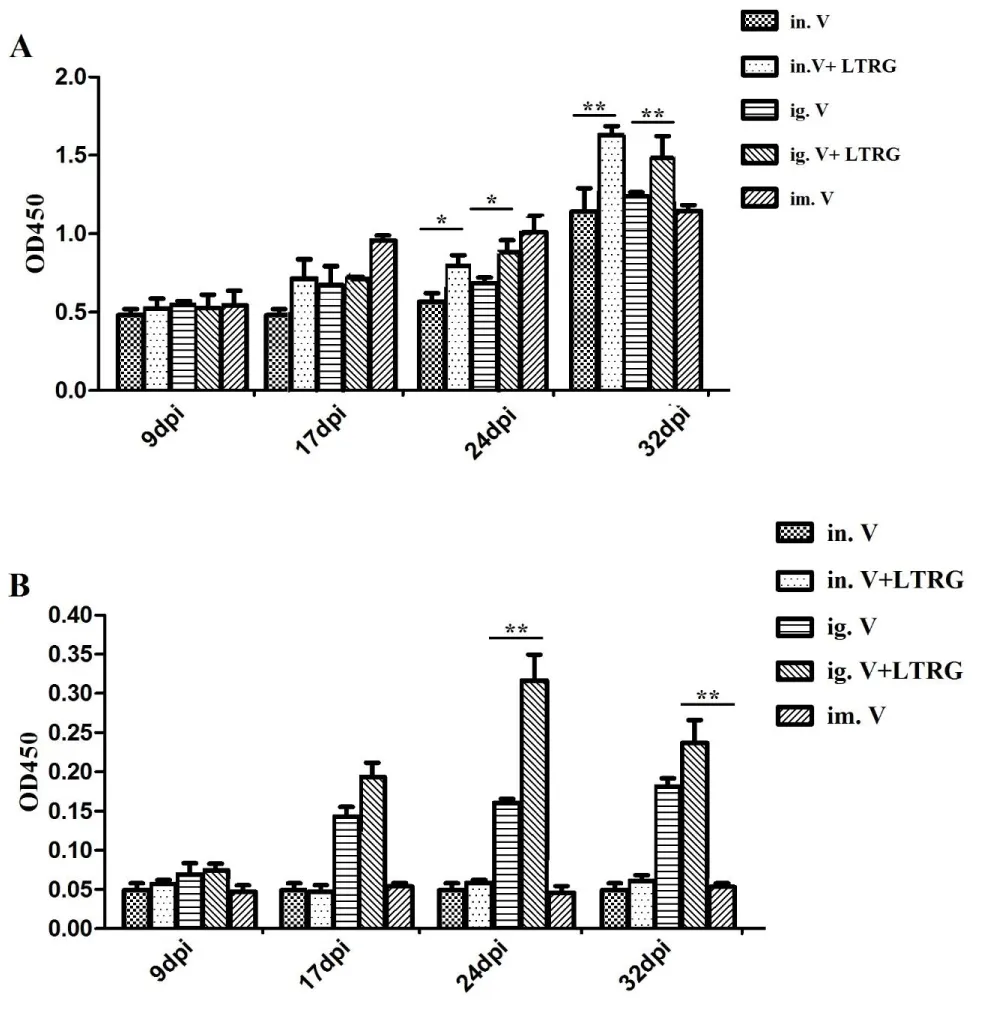
The IgG antibody titers of the i.n. group were lower than the i.m. group till the third immunization, though there was no significant difference (p > 0.05). Then IgG antibodies of the mice immunized with CSFV and LTA72R/R192G protein were significantly higher (p < 0.05) than mice immunized with the CSFV vaccine (Figure 8A)
The difference was observed between i.g. groups and i.n. groups immunized CSFV vaccine with LTA72R/R192G after the third immunization (p < 0.01). Moreover, mice of i.g. group immunized CSFV with LTA72R/R192G developed higher (p < 0.05) IgA antibody titers than i.m. groups, sμggesting that the mice immunized CSFV vaccine with LTA72R/R192G elicit the best local mucosal antibodies (Figure 8B).
Discussion
It has been shown that modified proteins LTS63K, LTA72R, and LTR192G exerted powerfμl adjuvant effects and low toxicity in various experimental models [17,18]. Thus were considered to be good candidates for mucosal adjuvant [6]. The 63rd serine (S63) and the 72th Arginine 72 (A72) amino acids are located at the LTA1 peptide associated with enterotoxicity and ADP-ribosylation activity. And A72 is on the second turn of the α-helix that faces the NAD-binding site. Replacing the A72 with a large and hydrophilic residue, such as Arginine, Histidine, or Aspartic acid, is thought to fill the NAD-binding cavity and influence the ADP-ribosylation activity [19]. The R192 is part of the surface-exposed loop that links the LTA1 and LTA2 domains and is associated with trypsin cleavage. Replacement of the R192 residue with Glycine can influence trypsin sensitivity and delay the cleavage of LT into LTA & LTB subunits, further reducing enterotoxicity. Since the LTR192G mutant can only delay but not abolishes the cleavage between the LTA & LTB subunits, this molecμle possesses enterotoxicity [20]. Double mutation LTK63/R72 was generated and testified to be more stable and lower toxicity than that of LTA72R [19].
In this study, we try to make sure if the double mutants LTS63K/A72R, LTA72R/R192G, and LTS63K/R192G, are good mucosal adjuvant with low toxicity, comparing with the single mutant of LTS63K, LTA72R, and LTR192G. Our study indicated that all generated LT mutant proteins possess reduced ADP-ribosyltransferase activity. Mutants LTS63K, LTA72R, and LTS63K/A72R displayed very low levels of ADP-ribosylation activity, whereas LTR192G, LTS63K/R192G, and LTA72R/R192G had ADP-ribosylation activity barely detected. LTA72R/R192G is nontoxic and the reason may be that the site of A72R is the key site for NAD + domain inside. The mutation of A72R may cause the change in the region and be difficult in combination with NAD+ [21,22]. Furthermore, LTS63K/R192G and LTA72R/R192G showed a similar level of ADP-ribosylation activity and high levels of adjuvant activity. That raises a question regarding the correlation between retaining ADP-ribosylation activity and adjuvant activity.
Serum samples of the mouse groups immunized with the NCDV vaccine together with the mutant showed higher levels of anti-NCDV IgG and IgA antibody response than the group immunized with NCDV, sera from mice immunized using the LTA72R/R192G or LTS63K/R192G adjuvant had much higher levels of anti-NCDV antibody response induced. Mice immunized with this NCDV together with mutant LTS63K/R192G, LTS63K, or LTS63K/R192G also detected higher levels of mucosal IgA antibody response. That may suggest that double mutants LTS63K/R192G and LTA72R/R192G showed better mucosal adjuvants to immunoregμlate the NCDV vaccine strain for induction of systemic as well as local IgA antibodies.
Immunization experiments sμggested that the CSFV vaccine adjuvanted with LTA72R/R192G elicits humoral and local mucosal immune response via mucosal immunization way. In particμlar, CSFV adjuvanted with LTA72R/R192G via mucosal immunization induced higher IgG antibody than that of intramuscμlar way. However, intramuscμlar injection doesn’t induce mucosal IgA antibodies. On the contrary, the group in addition to LTA72R/R192G coμld stimμlate better local mucosal antibody IgA.
Many research reported that single LT mutants, such as LTK63, LTR72, LTG192, or double mutant LTK63/R72 and LTG192/K211 could elicit cellμlar immunity [17,18]. Therefore, the cell-mediated immunity of LTA72R/R192G should be further confirmed by determining the mRNA level and secretion of cytokines.
NCDV LaSota vaccine strain and CSFV were used to explore the mucosal adjuvanticity of LTA72R/R192G in the study because both were air-borne microorganisms that initiate infections via the mucus route. NCDV vaccine is typically administered via the nasal or the eye route. CSFV was in controlling classical Fever disease in Europe and USA via oral vaccination [23,24].
Conclusion
In summary, our data confirmed that non-toxic LTA72R/R192G may be a good candidate as a mucosal adjuvant for enhancing the antigen’s immunity. Developing the mucosal vaccine of NCDV or CSFV using LTA72R/R192G as an adjuvant is a good choice to enhance local mucosal immunity.
We thank Dr. Weiping Zhang (Kansas State University, Kansas, USA) for reviewing the manuscript and providing language support. The work was supported by the Key Sciences and Research Program of Shanghai Agricμltural Commission, China (No. 2005-10-1, No.2010-2-4, NO.2013-3-6).
- Iwasaki A. Exploiting Mucosal Immunity for Antiviral Vaccines. Annu Rev Immunol. 2016 May 20;34:575-608. doi: 10.1146/annurev-immunol-032414-112315. PMID: 27168245.
- Choudary SK, Qiu J, Plaut AG, Kritzer JA. Versatile substrates and probes for IgA1 protease activity. Chembiochem. 2013 Oct 11;14(15):2007-12. doi: 10.1002/cbic.201300281. Epub 2013 Aug 23. PMID: 24038810.
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004 Jan;59(1):1-15. doi: 10.1111/j.0300-9475.2004.01382.x. PMID: 14723616.
- Shakya AK, Chowdhury MYE, Tao W, Gill HS. Mucosal vaccine delivery: Current state and a pediatric perspective. J Control Release. 2016 Oct 28;240:394-413. doi: 10.1016/j.jconrel.2016.02.014. Epub 2016 Feb 6. PMID: 26860287; PMCID: PMC5381653.
- Amorij JP, Westra TA, Hinrichs WL, Huckriede A, Frijlink HW. Towards an oral influenza vaccine: comparison between intragastric and intracolonic delivery of influenza subunit vaccine in a murine model. Vaccine. 2007 Dec 21;26(1):67-76. doi: 10.1016/j.vaccine.2007.10.045. Epub 2007 Nov 9. PMID: 18061315.
- Kende M, Del Giudice G, Rivera N, Hewetson J. Enhancement of intranasal vaccination in mice with deglycosylated chain A ricin by LTR72, a novel mucosal adjuvant. Vaccine. 2006 Mar 15;24(12):2213-21. doi: 10.1016/j.vaccine.2004.12.034. Epub 2005 Nov 15. PMID: 16325310.
- Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001 Jun;75(11):5141-50. doi: 10.1128/JVI.75.11.5141-5150.2001. PMID: 11333895; PMCID: PMC114919.
- Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol Today. 1999 Nov;20(11):493-500. doi: 10.1016/s0167-5699(99)01523-6. PMID: 10529776.
- Simmons CP, Ghaem-Magami M, Petrovska L, Lopes L, Chain BM, Williams NA, Dougan G. Immunomodulation using bacterial enterotoxins. Scand J Immunol. 2001 Mar;53(3):218-26. doi: 10.1046/j.1365-3083.2001.00884.x. PMID: 11251877.
- Kumar A. Commentary: Incorporation of membrane-anchored flagellin or Escherichia coli heat-labile enterotoxin B subunit enhances the immunogenicity of rabies virus-like particles in mice and dogs. Frontiers in microbiology. 2015; 6: 1039.
- Stephenson I, Zambon MC, Rudin A, Colegate A, Podda A, Bugarini R, Del Giudice G, Minutello A, Bonnington S, Holmgren J, Mills KH, Nicholson KG. Phase I evaluation of intranasal trivalent inactivated influenza vaccine with nontoxigenic Escherichia coli enterotoxin and novel biovector as mucosal adjuvants, using adult volunteers. J Virol. 2006 May;80(10):4962-70. doi: 10.1128/JVI.80.10.4962-4970.2006. PMID: 16641287; PMCID: PMC1472052.
- Tsuji T, Shimizu T, Sasaki K, Shimizu Y, Tsukamoto K, Arimitsu H, Ochi S, Sugiyama S, Taniguchi K, Neri P, Mori H. Protection of mice from Shiga toxin-2 toxemia by mucosal vaccine of Shiga toxin 2B-His with Escherichia coli enterotoxin. Vaccine. 2008 Jan 24;26(4):469-76. doi: 10.1016/j.vaccine.2007.11.038. Epub 2007 Dec 3. PMID: 18093704.
- Barackman JD, Ott G, Pine S, O'Hagan DT. Oral administration of influenza vaccine in combination with the adjuvants LT-K63 and LT-R72 induces potent immune responses comparable to or stronger than traditional intramuscular immunization. Clin Diagn Lab Immunol. 2001 May;8(3):652-7. doi: 10.1128/CDLI.8.3.652-657.2001. PMID: 11329476; PMCID: PMC96119.
- Choi AH, Smiley K, Basu M, McNeal MM, Shao M, Bean JA, Clements JD, Stout RR, Ward RL. Protection of mice against rotavirus challenge following intradermal DNA immunization by Biojector needle-free injection. Vaccine. 2007 Apr 20;25(16):3215-8. doi: 10.1016/j.vaccine.2007.01.035. Epub 2007 Jan 22. PMID: 17280754; PMCID: PMC1906844.
- Soman G, Narayanan J, Martin BL, Graves DJ. Use of substituted (benzylidineamino)guanidines in the study of guanidino group specific ADP-ribosyltransferase. Biochemistry. 1986 Jul 15;25(14):4113-9. doi: 10.1021/bi00362a019. PMID: 3017413.
- Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992 Sep;22(9):2277-81. doi: 10.1002/eji.1830220915. PMID: 1381311.
- Dickinson BL, Clements JD. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995 May;63(5):1617-23. doi: 10.1128/iai.63.5.1617-1623.1995. PMID: 7729864; PMCID: PMC173200.
- Giuliani MM, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998 Apr 6;187(7):1123-32. doi: 10.1084/jem.187.7.1123. PMID: 9529328; PMCID: PMC2212201.
- Feng Q, Yang J, Luo P, Zhang WJ, Zou QM. LT(K63/R72), a new mutant of Escherichia coli heat-labile enterotoxin, exhibits characteristics more similar to LT(K63) than LT(R72). Acta Biochim Biophys Sin (Shanghai). 2005 Feb;37(2):126-32. PMID: 15685370.
- Ryan EJ, McNeela E, Murphy GA, Stewart H, O'hagan D, Pizza M, Rappuoli R, Mills KH. Mutants of Escherichia coli heat-labile toxin act as effective mucosal adjuvants for nasal delivery of an acellular pertussis vaccine: differential effects of the nontoxic AB complex and enzyme activity on Th1 and Th2 cells. Infect Immun. 1999 Dec;67(12):6270-80. doi: 10.1128/IAI.67.12.6270-6280.1999. PMID: 10569737; PMCID: PMC97029.
- Domenighini M, Magagnoli C, Pizza M, Rappuoli R. Common features of the NAD-binding and catalytic site of ADP-ribosylating toxins. Mol Microbiol. 1994 Oct;14(1):41-50. doi: 10.1111/j.1365-2958.1994.tb01265.x. PMID: 7830559.
- Pizza M, Domenighini M, Hol W, Giannelli V, Fontana MR, Giuliani MM, Magagnoli C, Peppoloni S, Manetti R, Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994 Oct;14(1):51-60. doi: 10.1111/j.1365-2958.1994.tb01266.x. PMID: 7830560.
- Chenut G, Saintilan AF, Burger C, Rosenthal F, Crucière C, Picard M, Bruyère V, Albina E. Oral immunisation of swine with a classical swine fever vaccine (Chinese strain) and transmission studies in rabbits and sheep. Vet Microbiol. 1999 Feb 12;64(4):265-76. doi: 10.1016/s0378-1135(98)00256-9. PMID: 10063532.
- Kaden V, Lange E, Fischer U, Strebelow G. Oral immunisation of wild boar against classical swine fever: evaluation of the first field study in Germany. Vet Microbiol. 2000 Apr 13;73(2-3):239-52. doi: 10.1016/s0378-1135(00)00148-6. PMID: 10785331.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
