Journal of Vaccines and Immunology
Preparation and Application of two Monoclonal Antibodies against Canine Parvovirus Vaccine and Field Strains
Liying Hao1#, Ying Wang1#, Chenyu Bai1, Yudan Jin1, Dingding Zheng1, Lingyan Chen1, Xiangdong Li1, Junhua Deng1 and Kegong Tian1,2*
2College of Animal Science and Veterinary Medicine, Henan Agricultural University, Zhengzhou, 450002, P.R. China
#these authors contributed equally to this work
Cite this as
Hao L, Wang Y, Bai C, Jin Y, Zheng D, et al. (2017) Preparation and Application of two Monoclonal Antibodies against Canine Parvovirus Vaccine and Field Strains. J Vaccines Immunol 3(1): 001-004. DOI: 10.17352/jvi.000020Background: Canine parvovirus (CPV) emerged in 1970s as a highly infectious disease. CPV modified live vaccines have been widely used to control the disease. It is urgent to develop specific monoclonal antibodies to differentiate field virus from vaccine virus in vaccinated dogs.
Methods: In this study, female BALB/C mice were immunized with a commercial CPV vaccine strain. Two monoclonal antibodies (MAbs) 10H4 and 10B11 were made by hybridoma technique, and screened by hemagglutination inhibition (HI) assay.
Results: MAb 10H4 reacted with both CPV field and vaccine strains, and MAb 10B11 only recognized field but not vaccine strains by the results of HI, indirect immunofluorescence (IFA), and virus neutralization tests.
Conclusion: Therefore, these two MAbs may work as useful tools to study the CPV pathogenic mechanisms and to develop diagnostic reagents.
Abbreviations
CPV: Canine Parvovirus; CPV-2: CPV type 2; MAb: Monoclonal Antibody; MEV: Mink Enteritis Virus; FPV: Feline Parvovirus Virus; HA: Hemagglutination; HI: Hemagglutinin Inbitation; IFA: Indirect Immunofluorescence; VN: Virus Neutralization
Introduction
Canine parvovirus disease is an acute and highly infectious disease caused by canine parvovirus type 2 (CPV-2). The outbreak of canine parvovirus disease could be a seriously threat to the health of dogs, and also caused huge economic losses to the general economic animal breeding.CPV-2 was first recognized in the late 1970s, and then widely spread via genetic mutations and evolution with affecting different ages of dogs all over the world with infection rate from 50% to 100% and mortality of at least 50% [1-2]. Until the mid-1980s, CPV variants including CPV-2a and CPV-2b emerged in some countries with different pathogenicity [3-6]. In the past 30 years, CPV-2a and CPV-2b have mainly spread in different proportions among dog populations throughout the world, and then CPV new variants (CPV-2c) were detected in Europe, Asia, and North and South America [7-9]. Up to now, except a product containing modified-live CPV-2b strain (Galaxy DA2PPv; Schering-Plough Animal Health), CPV strain subtype of the other commercial vaccines was CPV-2a such as Nobivac®DHPPi, Progard®-CPV, Vanguard®Plus 5, Recombitek® Canine Parvo and “Rabies, Canine Distemper, Parainfluenza, Adenovirus and Parvovirus Vaccine for Dogs, Live” Vaccine. Therefore, monoclonal antibodies that can differentiate the vaccine strains from field strains of CPV will be valuable.
MAbs work as a useful tool to study the CPV pathogenic mechanisms and to develop corresponding diagnostic reagents. The principal objective of present study is to screen CPV MAbs by using hybridoma techniques and generate MAbs with good reactivity of CPV vaccine and/or field strains.
Materials and Methods
Cells and viruses
CPV strain CVCC AV298 was purchased from China Veterinary Culture Collection Center. CPV-2a field strains S2 and S0425, and CPV-2b field strain S0304 were isolated, identified and stored in our laboratory. CPV-2a vaccine strain S0425-vaccine was obtained by continuous culturing CPV-2a strain S0425 on F81 cells for 80 passages. Nobivac®DHPPi (Intervet International B.V.), Vanguard®Plus 5 (Pfizer Animal Health) and a domestic vaccine (“Rabies, Canine Distemper, Parainfluenza, Adenovirus and Parvovirus Vaccine for Dogs, Live” Vaccine, Five-Star Animal Health Pharmaceutical Factory of Jilin Province) were purchased, and the above CPV vaccine strains belong to CPV-2a type.
Animals and Immunization of mice
BALB/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., and then kept in the animal facility. For the first immunization, a group of six-weeks old female BALB/c mice (n=4) were injected with 200μl CPV strain CVCC AV298 plus equal volume of complete Freund’s adjuvant (Sigma-Aldrich, Missouri, United States) via hypodermic injection at four different sites on the back. Once every two weeks, another hypodermic immunization was administrated by using 200μl CPV Strain CVCC AV298 emulsified in a 1:1 ration in incomplete Freund’s adjuvant (Sigma-Aldrich, Missouri, United States). After three immunizations, the equal amount of antigen without adjuvant was injected into mice through intraperitoneal injection for the boost. One mouse without immunization worked as control.
Preparation of anti-canine parvovirus MAbs
In order to prepare MAbs, SP2/0 myeloma cells were in advance cultured with RPMI1640 medium (Gibco, Grand Island, NY) containing of 17% fetal bovine serum (FBS, Hyclone, Logan, UT) and antibiotics (100U/ml penicillin and 100μg/ml streptomycin).
After multiple immunizations, the anti-CPV serum titer of mice was determined by using Hemagglutinin Inbitation (HI). Three days after the final injection, the spleen cells from the euthanized mice of the highest anti-CPV serum antibody titer were collected and fused with SP2/0 cells by using 50% Polythylene Glycol 1500 (PEG 1500; Roche Biochemicals, Indianapolis, IN, USA) as a fusion agent. The hybridoma cells were cultured in 96-well plates (Costar, Cambridge, MA) in a humidified incubator (Suzhou Antai Airtech co., Ltd.) containing 5% CO2 at 37°C in HAT (namely Media Supplement (50×) Hybri-Max, Purchased from Sigma-Aldrich, Missouri, United States) screening culture medium. The supernatant of the fusion cells was then subjected to HI test to detect anti-CPV antibody production. Positive hybridomas were selected and cloned for at least three times by limiting dilution, and injected into paraffin treated BALB/c mice (purchasing from Beijing Vital River Laboratory Animal Technology Co., Ltd.) for the purpose of gaining abundant ascetic fluid. Two MAbs (consisting of 10H4 and 10B11) were identified, and their isotypes were determined by the Pierce Rapid ELISA Mouse MAb Isotyping Kit (Thermo Scientic) according to the manufacturer’s instructions.
Purification of MAbs
Two MAbs were respectively purified from ascetics by affinity binding with protein G resin. In brief, 5 ml ascite plus 5ml binding/wash buffer (containing of 20mM Na2HPO4 and 0.15 M NaCl, pH8.0) was mixed and applied for equilibrating 5 ml column of protein G. One ascetic fluid was then needed to the column for binding. After washing the column with 30ml binding/wash buffer, the purified MAb was eluted by appropriate volume of elute buffer (0.1M glycine, pH2.5) and collected the Elute and immediately neutralize to pH7.4 with Neutralization Buffer (1M Tris-HCl, pH8.5). The purity of MAbs was measured by SDS-PAGE (Bio-Rad Laboratories, Inc.) and the concentration of MAbs was measured by BCA Protein Assay Kit (Beyotime Biotechnology, China).
Determination of relative affinity of MAbs
The relative affinity of MAbs was determined by indirect ELISA using thiocyanate.
In brief, ascites of 10H4, 10B11 and SP2/0 cells were diluted with different concentrations of NaSCN and OD 450 was measured to evaluate the reactivity between antigen and antibody. The concentration of NaSCN was considered as the relative affinity Ka of 10H4 and 10B11 when the OD 450 value declined by 50% as much as that when it was not eluted. The unit of relative affinity is expressed by mol/L.
Hemagglutination inhibition (HI) assay
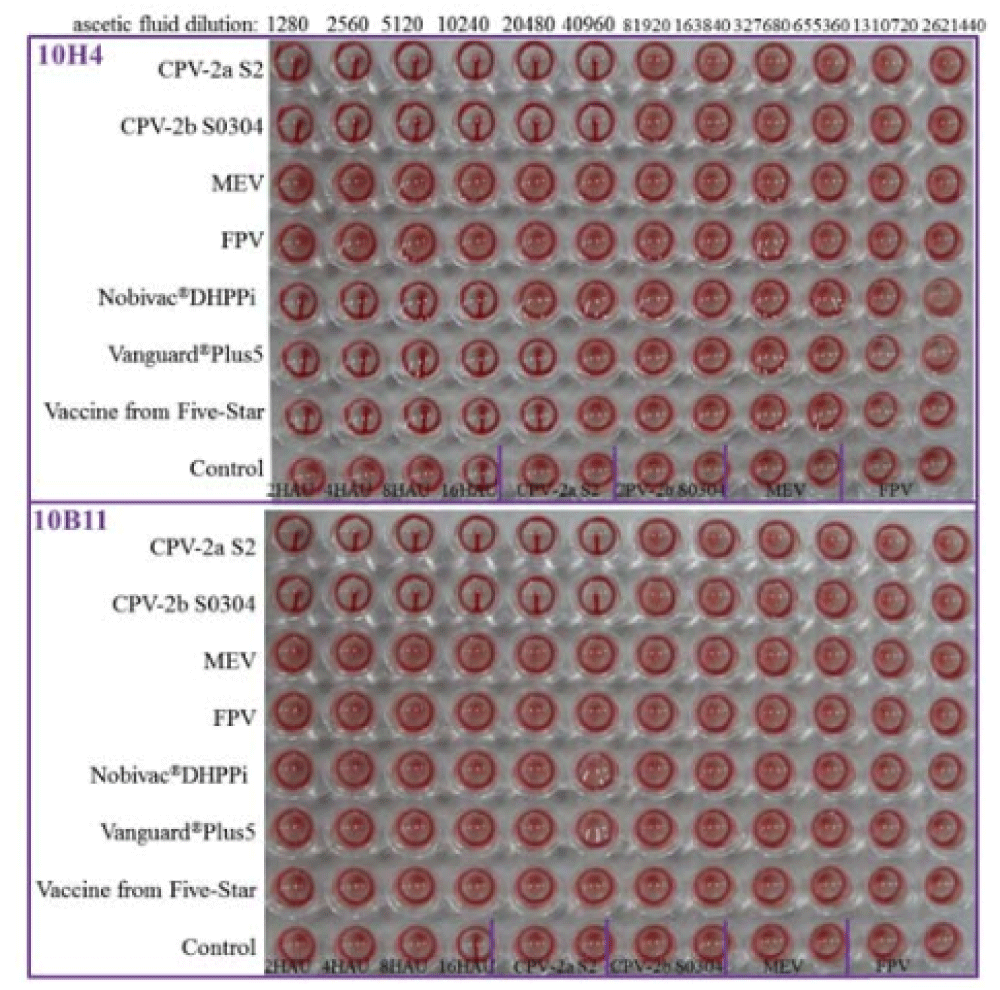
HI assay was followed as described previously [10]. Phosphate-buffered saline (pH6.4) was used for the HI buffer, and ascetic fluid was firstly diluted to 1:10. Subsequently, 2-fold serial dilutions of 1:10 ascetic fluid (25μl) was mixed with viruses (8 hemagglutination units, HAU/25μl), and incubated at 37°C for 30min. At the same time, CPV/MEV/FPV antigen and red blood cell control wells were tested. Then, added 50μl of HI buffer containing of 1% porcine erythrocytes and mildly shook blending before setting at 4 for 2h. The HI titer was expressed as the reciprocal of the highest dilution which completely inhibited viral hemagglutination.
MEV and FPV (abbreviation for Mink enteritis and Feline Parvovirus virus, stored in our laboratory) were simultaneously detected by HI tests for verifying two MAbs’ specificity.
Indirect immunofluorescence (IFA)
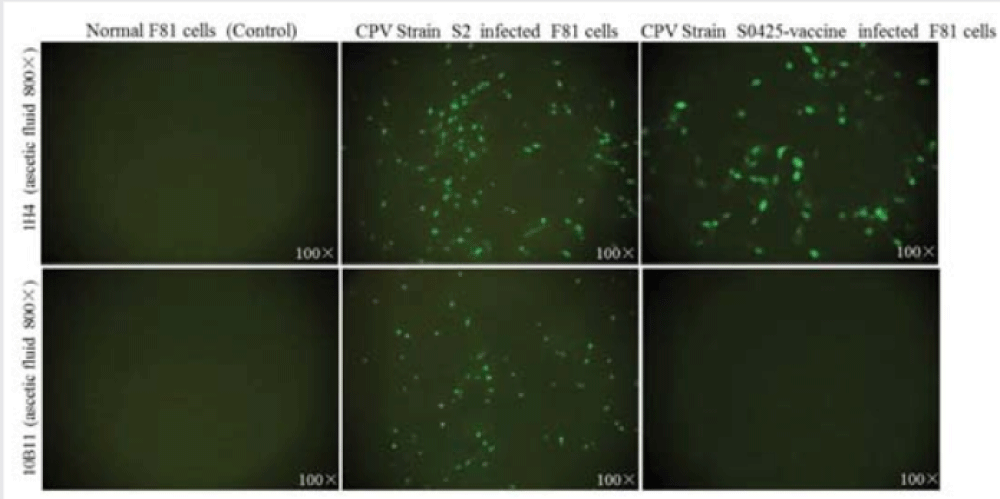
IFA was used to detect the reactivity of two MAbs to CPV. Feline kidney F81 cells were inoculated with CPV-2a strains S2 or C154, and were cultured in incubate or in a 5 % carbon dioxide and 95% atmosphere at 37°C, with minimal essential medium (MEM, HyClone) containing 5% fetal bovine serum (HyClone). After 48h, when CPE appeared, discarded the medium and digested the infected F81 cells with trypsin. Next, fixed the cells in the well of 96-well plates with cold acetone for 30min at 4°C. Two MAbs’ ascite, negative serum from the mouse control, or commercial anti-CPV polyclonal serum (Beijing Springup Scientific Co., Ltd) were diluted with PBS (0.01mol/l, pH7.2) and added into the fixed F81 cells with 100μl/well, and incubated at 37°C for 1h. After washing three times, followed by addition 1:200 dilution of FITC-conjugated goat antimouse (Sigma, 100μl/well) and incubated for at 37°C 50min. After another washing three times, added PBS (50μl/well) and observe under fluorescence microscopy.
Virus neutralization (VN) tests
VN tests were performed as described previously [11]. In brief, 50μl 2-fold serial dilutions of ascetic fluid from 1:100, and viruses (100TCID50/50μl, with 4 replicates of Strains S2, S0425, S0304 or S0425-vaccine) were respectively mixed and incubated at 37°C for 1h. After that, add F81 cells (104cells/100μl) into this mixture. The cells were examined for 5 days, and the VN titers were expressed as the reciprocal of the highest dilution which inhibited the viral cytopathic effect in at least 2/4 wells with the particular dilution.
Results
Generation of MAbs against CPV
Compared with the unimmunized mouse, HI results showed that the antibody titers of fourth immunized mice ranged from 1:2560 to 1:5210. The mouse whose HI titer was 1:5210, was used for splenic cell isolation. After fusion with SP2/0 myeloma cells, two positive hybridoma clones (designated 10H4 and 10B11) were identified by HI assay. The cell chromosome count and isotypes of two MAbs were summarized in table 1. The relative affinity Ka of MAb 10H4 and 10B11 were 3.5mol/L and 4.0 mol/L, respectively.
Specificity and reactivity of two MAbs
In order to detect the reactivity and HI titer of two MAbs, HI assay was performed. As shown in figure 1, both of two MAbs (10H4 and 10B11) reacted with CPV field strains 2a and 2b with HI titers at 1:40960 without cross-reactivity of MEV and FPV. By contrast, MEV/FPV antigen and red blood cell control had no reactivity. MAb 10H4 was reactive with three vaccine CPVs (Nobivac®DHPPi, Vanguard®Plus 5, and Five star) with HI titers of 1:10240-1:20480, but MAb 10B11 was not.
IFA was utilized for detecting the presence of antibodies to CPV, and the results were shown in figure 2. IFA titers of MAb 10H4 from ascetics were 1:6400 to CPV field strain S2 and 1:3200 to CPV vaccine Strain (S0425-vaccine). By contrast, IFA titers of MAb 10B11 were 1:3200 to CPV field strain S2 and negative to CPV vaccine strain (S0425-vaccine). Normal F81 cells had no such reactivity.
Neutralizing capacity of two MAbs
The VN titers of 10H4 and 10B11 were summarized in table 2. The VN titers of 10H4 to CPV2a, CPV2b, and CPV vaccine were 1:12800~25600, 1:12800, and 1:6400, respectively. The VN titers of 10B11 to CPV2a and CPV2b were 1:12800 and 1:6400. Consistent with HI and IFA results, 10B11 had no reactivity with CPV vaccine virus.
Discussion
Monoclonal antibodies to different genotypes CPV were previously reported. In Masato Nakamura’s study, MAbs 2G5 and 20G4 recognized CPV-2a, CPV-2b, and CPV-2c, MAb 21C3 only reacted with CPV-2b and CPV-2c, and MAb 19D7 recognized all types of the FPV subgroup viruses [12]. However, there were no reports about any MAbs which could differentiate CPV vaccine from field viruses.
In this study, we are the first group to report that the MAb 10B11 could only react with CPV field virus by the results of HI, IFA, and VN tests. By contrast, another MAb 10H4 reacted with both CPV field viruses and vaccine strains. Differentiating the vaccinated dogs or CPV vaccine strains from the infected dogs or CPV field strains is very important for clinical measurement. The present widely-used techniques to discriminate between vaccine and field viruses were PCR and real-time PCR, but these methods are expensive and time-consuming [13,14]. The two MAbs in this study provide good biological reagents to develop high throughput and sensitive method such as ELISA to differentiate the vaccine from field viruses.
10H4 and 10B11 were generated by immunization of mice with CVCC AV298, a vaccine strain of CPV. The proteins that these two antibodies recognized need to be explored in the future study. Since both MAbs showed very high neutralizing antibodies to different genotypes of field CPVs, they may also work as therapeutic MAbs to treat CPV infection.
Conclusion
To conclude, two CPV MAbs were characterized in this study which MAb 10H4 reacted with both field and vaccine strains of CPV, Whereas, MAb 10B11 only reacted with field strain but not vaccine strain of CPV. These CPV MAbs may provide valuable biological reagents to study the CPV pathogenic mechanisms as well as to work as therapeutic antibodies.
This work was supported by grant from Luoyang Heluo Talent Plan (Dr. Kegong Tian).
- Parrish CR, Aquadro CF, Carmichael LE (1988) Natural variation of canine parvovirus Science 230: 1046-1048. Link: https://goo.gl/9a05DA
- Burtonboy G, Coignoul F, Delferrière N, Pastoret PP (1979) Canine hemorrhagic enteritis: detection of viral particles by electron microscopy Arch Virol 61: 1–11. Link: https://goo.gl/XfQ40U
- Carmichael LE, Schlafer DH, Hashimoto A (1994) Minute virus of canines (MVC, canine parvovirus type-1): pathogenicity for pups and seroprevalence estimate J Vet Diagn Invest 6: 165–174. Link: https://goo.gl/2Gm4I3
- Parrish CR, Aquadro CF, Strassheim ML, Evermann JF, J Y Sgro, et al. (1991)Rapid antigen-type replacement and DNA sequence evolution of canine parvovirus J Virol 65: 6544–6552. Link: https://goo.gl/3gTdlW
- Parrish CR, Have P, Foreyt WJ, Evermann JF, Megumi Senda, et al. (1988) the global spread and replacement of canine parvovirus strains J Gen Virol 69: 1111–1116. Link: https://goo.gl/tbmLMj
- Pereira CAD, Monezi TA, Mehnert DU, Angelo MD, Edison LD, et al. (2000) Molecular characterization of canine parvovirus in Brazil by polymerase chain reaction assay. Vet Microbiol 75: 127–133. Link: https://goo.gl/g6UceA
- Truyen U (2006) Evolution of canine parvovirus-a need for new vaccines? Vet Microbiol 117: 9–13. Link: https://goo.gl/DlVQhZ
- Buonavoglia C, Martella V, Pratelli A, Tempesta M, Alessandra Cavalli, et al. (2001) Evidence for evolution of canine parvovirus 2 in Italy J Gen Virol 82: 3021–3025. Link: https://goo.gl/ZtDQ0A
- Colin RP (1999) Host range relationships and the evolution of canine parvovirus Vet Microbiol 69: 29-40. Link: https://goo.gl/et31ue
- Mathys A, Mueller R, Pedersen NC, Theilen GH (1983) Hemagglutination with formalin-fixed erythrocytes for detection of canine parvovirus Am J Vet Res 44: 150–151. Link: https://goo.gl/gSY2dB
- Ikeda Y, Miyazawa T, Kurosawa K, Naito R, Shinichi H, et al. (1998) New quantitative methods for detection of feline parvovirus (FPV) and virus neutralizing antibody against FPV using a feline T lymphoid cell line J Vet Med Sci 60: 973–974 . Link: https://goo.gl/iiqoTI
- Masato N, Kazuya N, Takayuki M, Yukinobu T, Masami M, et al. (2003) Monoclonal Antibodies That Distinguish Antigenic Variants of Canine Parvovirus Clin Diagn Lab Immun 10: 1085-1089. Link: https://goo.gl/3yTmbZ
- Decaro N, Buonavoglia C (2012) Canine parvovirus-A review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet Microbiol 155: 1–12. Link: https://goo.gl/OL2nfT
- Senda M, Parrish CR, Harasawa R, Gamoh K, Muramatsu M, et al. (1995) Detection by PCR of Wild-Type Canine Parvovirus Which Contaminates Dog Vaccines. J Clin Microbiol 33: 110–113
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
