Journal of Vaccines and Immunology
Post-vaccinal reactions following vaccination with the COVID-19 vaccine after first and second dose
Velina Stoeva1*, Rosen Mihaylov2, Blagovesta Pencheva2 and Antoineta Mihova3
2Clinical Laboratory Specialist, SMDL Ramus Ltd., Sofia, Bulgaria
3UH Lozenets Laboratory, Clinical Immunology, Bulgaria
Cite this as
Stoeva V, Mihaylov R, Pencheva B, Mihova A (2024) Post-vaccinal reactions following vaccination with the COVID-19 vaccine after first and second dose. J Vaccines Immunol 10(1): 006-010. DOI: 10.17352/jvi.000060Copyright
© 2024 Stoeva V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Among the countries in the European Union, Bulgaria reports the lowest immunization coverage. Gathering reliable information on the level of post-vaccine adverse events and making it publicly available might increase public confidence in vaccines and immunization strategies. The aim of our study is to analyze and summarize post-vaccination reactions among 100 pre-hospital care patients after the administration of the first and second doses of the SARS-CoV-2 vaccines. The reported adverse reactions found by us with both mRNA and adenoviral vector vaccines were mild and self-limiting, similar to the results reported by many other authors in different parts of the world. We hope that attitudes regarding vaccinations will change in favor of vaccines, precisely through the analysis of the results of post-vaccination side effects, proof of their harmlessness.
Regarding vaccinations will change in favor of vaccines, precisely through the analysis of the results of post-vaccination side effects, proof of their harmlessness.
Introduction
The pandemic spread of the Coronavirus disease (COVID-19) has put the health of people around the world in an extraordinary and difficult-to-control situation. The causative agent, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is highly contagious and has caused over a million deaths since its discovery in Wuhan, China in December 2019. The first patients likely became infected at a live animal market in the city.
Due to the global spread of SARS-CoV-2, the World Health Organization (WHO) declared a pandemic on March 11, 2020 [1-4]. In many cases, infections caused by SARS-CoV-2 are mild or asymptomatic, but in the elderly and those with chronic health problems, they can lead to severe COVID-19, requiring invasive ventilation, or death [5-7].
Globally, the incidence of COVID-19 manifests itself with intense epidemic waves, etiologically related to the new variants of the mutating coronavirus.
The pandemic spread of SARS-CoV-2 has presented a huge public health challenge. Despite the widespread application of science-based prevention and control measures, the expected rapid control of the spread of the virus has not been achieved.
At the beginning of the Pandemic, morbidity, both in our country and around the world, was much more intense, and now, probably due to people’s awareness of the prevention of infection, vaccination, and compliance with a much stricter anti-epidemic regime, a significant decrease is noticeable.
Historically, the absolute controlling means of dealing with epidemics is the vaccination of the population, with the goal being the formation of collective immunity. Collective immunity, as is known, is achieved by vaccination in over 70% of the population - such values were not achieved in Bulgaria.
There is a lot of data in the literature about the protective role of immunity that builds up after vaccination, its tension over time, etc.
Restoring public confidence in vaccination such as, and in this case building sustained confidence in SARS-CoV-2 vaccines, is critical to the implementation of a successful immunization program. Collecting and making available to the population in a clear and easy-to-understand form reliable information about adverse reactions after immunization, as well as their severity, or lack thereof, can increase people’s awareness and reasonably encourage them to get a vaccine against COVID-19.
Purpose
To analyze, evaluate, and summarize the conditions of the COVID-19 pandemic and the vaccination-related post-vaccination reactions, because vaccines are an important and reliable controlling pandemic factor.
Materials and methods
In the period August 2021- August 2022, a cross-sectional study was conducted among 100 people who were vaccinated with one of the vaccines allowed in Bulgaria. The survey was conducted in the largest privately-owned laboratory in Bulgaria “SMDL Ramus Ltd.”. The facilities in several cities were included (Sofia, Plovdiv, Yambol). The study received approval from the Ethical Committee of the Medical University of Plovdiv, Bulgaria (Protocol 4/08.06.2022). The research was conducted in compliance with the Declaration of Helsinki.
The sample size was not pre-determined. Rather, this was the number of subjects that were available and responded positively to the vaccination and the participation in this study for the allowed duration for sample collection. Our purpose was to collect pilot data facilitating the choice of design for further investigations.
A paper-based questionnaire was provided to the participants prior to blood sampling and after the informed consent form signing. The subjects were thoroughly informed about the potential risks and benefits in relation to their participation in this survey and that there were no additional expenses for them. The questionnaire contained the following sections:
- Demographics, occupation, comorbidities of participants, and information on previous COVID-19 infections and adverse reactions following immunization in the past.
- Information about the first and second doses of the vaccine against COVID-19, i.e. the type of vaccine, the occurrence of adverse events after immunization - local reactions, systemic reactions, allergic reactions, and other reactions (thrombocytopenia, blood disorders, syncope, arthralgia).
The eligibility criteria for participants to be included in the study are:
- Age ≥ 18 years
- Vaccination with any of the available vaccines, regardless of previous infection with COVID-19
- Written consent for voluntary participation without financial compensation and the possibility of refusal at any time until the submission of the data.
The exclusion criteria included
- Age under 18 years
- Subjects who were not able or willing to provide three blood samples for the survey period
- Pregnant and breastfeeding women
Statistical considerations
The collected data was analyzed using the Pearson test and the methods of descriptive statistics. The statistical package SPSS 26 was used.
Results
Follow-up and analysis of post-vaccination reactions in pre-hospital care patients after the administration of the first and second doses of the SARS-CoV-2 vaccines.
The mean age of study participants was 49 years (37 years; 60 years). Female respondents predominated (n = 60; 60%). During the study period, information was collected and analyzed for 76 respondents vaccinated with two doses of Pfizer-Biontech or Moderna mRNA vaccine and 24 respondents vaccinated with one dose of Jannsen vector vaccine, respectively.
A total of 69 (69.0%) of participants reported at least one local reaction after the first vaccine dose, and the prevalence among the mRNA-based vaccine group was higher (72.4%) than in the viral vector-based vaccine group (58.3%) but did not reach statistical significance (Pearson χ2 test = 1.680, p = 0.195). The most common local adverse reactions after vaccination with both vaccines were pain at the injection site (72.4%) for mRNA-based and viral vector-based vaccines (58.3%). The majority of respondents rated the pain as mild (66.7% for mRNA-based vaccine and 71.4% for viral vector-based vaccine). In terms of time of onset, most participants reported experiencing pain at the injection site around 24 hours after vaccine administration (66.7% for mRNA-based vaccine and 57.1% for viral vector-based vaccine).
80.2% of respondents reported local reactions after a second dose of the mRNA vaccine. As with the first dose, the main local reaction was pain at the injection site 80.2%. The majority of respondents defined the pain as mild - 91.8% and occurring 24 hours after administration of the second dose of vaccine - 86.9%.
A total of 35 (35%) of participants reported at least one systemic reaction after administration of the first dose of the COVID-19 vaccine. The prevalence of systemic reactions among the viral vector-based vaccine group was higher (41.7%) than in the mRNA-based vaccine group (32.8%) but did not reach statistical significance (Pearson χ2 test = 0.617, p = 0.432). The most commonly reported systemic reaction after the first dose of
COVID-19 vaccine fatigue (60% among mRNA vaccinees and 50% among vector vaccinees). Respondents most often reported that fatigue occurred in the first 24 hours after vaccination and was mild in severity without impairing the performance of daily activities.
After the second dose of the mRNA vaccine, 39.5% of respondents reported a systemic reaction, with again the most commonly reported reaction being post-vaccine fatigue - 60% occurring within the first 24 hours post-vaccination and mild in severity.
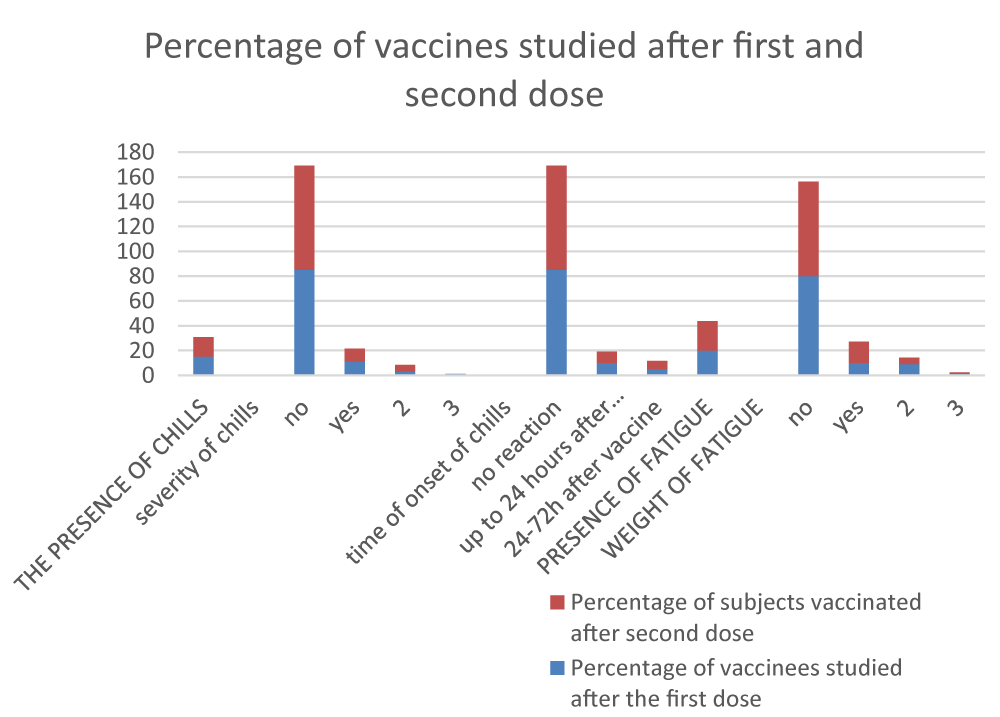
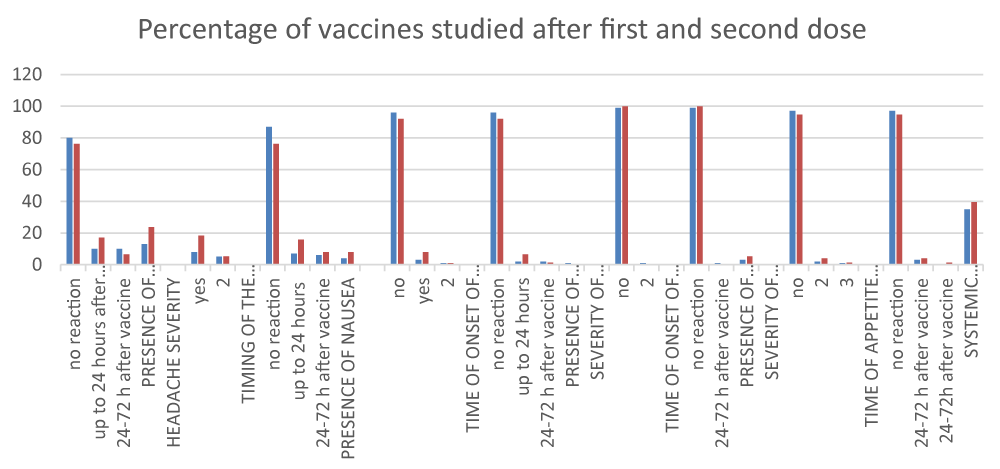
Data from the first group of local reactions are presented in (Figure 1). The presence of pain was found in 69% after the first and 80% after the second immunization, with mild or moderate reactions prevailing. Edema is reported from 7% at the first dose and from 3% at the second. Edema severity was again mild to moderate and occurred up to a maximum of 3 days after immunization. There is also evidence of mild to moderate redness in only about 5% reported by the 72nd hour.
From the obtained results, it can be concluded that although a high percentage of people note the presence of pain during vaccination, it is not severe and is very rarely combined with swelling and redness. Such local reactions have been reported with other immunization regimens and do not alter the rhythm and quality of life of vaccinated individuals.
Summary results of the second group of local reactions, covering possible rashes, muscle and joint pain, and the occurrence of fever are presented in (Figure 2). A rash was reported in only one person after the first dose. Muscle pain was reported in 13% and 19% of the group, respectively, covering a period of up to 24 hours and up to 72 hours after immunization. Joint pain was noted in 8% after the first and 20% after the second vaccination in the same time frames. The survey cards showed that 83% and 68%, respectively, did not have an increase in temperature. The temperature reported in the other persons was between 37 and 38 degrees, only one person reported a higher one - up to 38.5 degrees. The noted increase in temperature is mainly up to the 24th hour after immunization.
The results also in this group of local reactions do not differ in terms of frequency and severity from other post-vaccination reactions.
Another group of reactions reflected by project participants are systemic reactions after vaccination. Such reactions were found in 35% and 39% after the first and second doses, respectively. It was found that a high percentage of the group (85%-80%) did not experience chills, fatigue, and nausea. A very small proportion of the vaccinated reported nausea after the first and second vaccinations, respectively 4% and 8%, diarrhea – 1% after the first vaccination, and decreased appetite in 3% and 5.3%.
No nodule, infected or sterile abscess, or cellulitis were found after analysis of systemic reaction data.
Other possible reactions such as thrombocytopenia, arthritis/arthralgia, syncope (loss of consciousness), coagulation disorder, and other unusual events have not been identified.
Discussion
Detailed and comprehensive studies of the safety and efficacy of COVID-19 vaccines can reduce concerns in the general population about adverse reactions and the duration of protection they can provide.
Public confidence in vaccines can be improved by learning from past mistakes, and by creating an ongoing forum for providing new and up-to-date information and discussing new concerns as they arise.
Our study had some limitations, mainly in relation to the sample size that we were able to comply with for the survey duration. The main challenge for our team was to conduct the survey while the confidence in the vaccination was at its lowest point in Bulgaria. In addition, in the survey period, there was a rapid shift of the suggested laboratory assays for antigens and antibodies, all of which with a different reference range, which further challenged the patient trust in vaccination. In conclusion, the vaccination rate in Bulgaria remained low in comparison with the other EU countries.
A study of approximately 1,736 individuals reported results similar to those obtained by our team. The team aimed to identify adverse effects associated with three types of vaccines against coronavirus disease 2019. Participants included in the study were individuals who received the first dose or the full course (two doses) of the vaccine at least 30 days before the study. Vaccine reactogenicity, including pain, redness, urticaria, and swelling at the injection site, was reported in 34.56% of participants. A local site reaction was reported in more individuals who had the Pfizer and AstraZeneca vaccines than those who received the Sinopharm vaccine. Systemic events were more common with the AstraZeneca and Pfizer vaccines, reported symptoms were fatigue, body aches, headache, muscle aches, fever, and gastrointestinal side effects. There were no correlations between age or gender and the duration of adverse effects for the three vaccines [8].
Another team is conducting a study aiming to systematically review and synthesize the evidence regarding safety data from published COVID-19 vaccine trials. They searched three major electronic databases (PubMed, Embase, and Google Scholar) for studies published between December 2019 and 2020. Most of the reported reactions they found were mild to moderate, but some were of serious intensity. All reactions resolved within 3 days - 4 days. Commonly reported local adverse reactions were injection site pain, swelling, and redness. Systemic reactions have included fever, fatigue, myalgia, and headache. None of these changes are clinically apparent and are self-limiting. A few clinical trials reported serious side effects, but they were not related to the vaccination. This systematic review shows that vaccines against COVID-19 can be safe without serious side effects. However, long-term post-marketing surveillance data, especially in high-risk vulnerable groups (elderly and those with co-morbidities, pregnant women and children), are warranted to ensure the safety of vaccines against COVID-19 [9].
The study by a team from Saudi Arabia aimed to perform a systematic review of the four vaccines against COVID-19 (AstraZeneca, Pfizer, Moderna, and Janssen) approved in Saudi Arabia. The study was conducted by reviewing published articles from electronic databases such as PubMed, Embase, Cochrane Library, and Web of Science. The review analyzed eighteen articles and their data were evaluated to analyze the safety and efficacy of vaccines in different population groups such as men, women, people over 18 years, and people with co-morbidities. Common local reactions observed after vaccination are injection site pain (40% - 70%), redness (16% - 30%), swelling (18% - 39%) and tenderness (20% - 40%). Systemic reactions reported are fever (40% - 60%), chills (12% - 23%), fatigue (44% - 65%), headache (30% - 42%) and muscle pain (15% - 40%). The efficacy was found to be above the threshold value (60%) set by WHO. However, precautions should be taken when vaccinating special populations, such as those who are pregnant, nursing, or suffering from a serious illness. Furthermore, the rare and serious adverse events reported remotely after vaccination need more studies [10].
The efficacy of the vaccinations was not the target aim of this survey.
Conclusion
The reported adverse reactions we found with both mRNA and adenoviral vector vaccines were mild and self-limiting, similar to results reported in other parts of the world. The reported reactions were expected according to the Summary of Product Characteristics, and no adverse reactions were reported that were not listed in the information for the approved vaccines. All three vaccines did not cause severe side effects.
The reported post-vaccination reactions are relevant to confirm the areactogenicity of the vaccines used, which is motivating for the hesitant. We expect that attitudes regarding vaccinations will change in favor of vaccines, precisely through the analysis of the results of post-vaccination adverse reactions, proof of their harmlessness.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270-273. doi: 10.1038/s41586-020-2012-7. Epub 2020 Feb 3. Erratum in: Nature. 2020 Dec;588(7836):E6. PMID: 32015507; PMCID: PMC7095418. https://pubmed.ncbi.nlm.nih.gov/32015507/
- WHO Director-General's opening remarks at the media briefing on COVID-19. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing---5-may-2023
- Bourouiba L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA. 2020 May 12;323(18):1837-1838. doi: 10.1001/jama.2020.4756. PMID: 32215590.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations: scientific brief, 29 March 2020. World Health Organization. 2020. https://iris.who.int/handle/10665/331616. License: CC BY-NC-SA 3.0 IGO
- I. Meyts, G. Bucciol, I. Quinti, B. Neven, A. Fischer, E. Seoane, E. Lopez-Granados, C. Gianelli, A. Robles-Marhuenda, P. Y. Jeandel, C. Paillard, V. G. Sankaran, Y. Y. Demirdag, V. Lougaris, A. Aiuti, A. Plebani, C. Milito, V. A. Dalm, K. Guevara-Hoyer, S. Sánchez-Ramón, L. Bezrodnik, F. Barzaghi, L. I. Gonzalez-Granado, G. R. Hayman, G. Uzel, L. O. Mendonça, C. Agostini, G. Spadaro, R. Badolato, A. Soresina, F. Vermeulen, C. Bosteels, B. N. Lambrecht, M. Keller, P. J. Mustillo, R. S. Abraham, S. Gupta, A. Ozen, E. Karakoc-Aydiner, S. Baris, A. F. Freeman, M. Yamazaki-Nakashimada, S. Scheffler-Mendoza, S. Espinosa-Padilla, A. R. Gennery, S. Jolles, Y. Espinosa, M. C. Poli, C. Fieschi, F. Hauck, C. CunninghamRundles, N. Mahlaoui, K. Warnatz, K. E. Sullivan, S. G. Tangye; IUIS Committee of Inborn Errors of Immunity, Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J. Allergy Clin. Immunol. 2020. S0091- 6749(20)31320-8. doi:10.1016/j.jaci.2020.09.010 Medline https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-792893
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30; PMID: 31986264; PMCID: PMC7159299.
- Nguyen-Contant P, Embong AK, Kanagaiah P, Chaves FA, Yang H, Branche AR, Topham DJ, Sangster MY. S Protein-Reactive IgG and Memory B Cell Production after Human SARS-CoV-2 Infection Includes Broad Reactivity to the S2 Subunit. mBio. 2020 Sep 25;11(5):e01991-20. doi: 10.1128/mBio.01991-20. PMID: 32978311; PMCID: PMC7520599.
- Al Khames Aga QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahham AT, Shaban D, Al Khames Aga LA, Agha MYR, Traqchi M. Safety of COVID-19 vaccines. J Med Virol. 2021 Dec;93(12):6588-6594. doi: 10.1002/jmv.27214. Epub 2021 Jul 28. PMID: 34270094; PMCID: PMC8426829.
- Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, Singh K, Yadav D, Sharma P, Misra S. Adverse Events Reported from COVID-19 Vaccine Trials: A Systematic Review. Indian J Clin Biochem. 2021 Oct;36(4):427-439. doi: 10.1007/s12291-021-00968-z. Epub 2021 Mar 27. PMID: 33814753; PMCID: PMC7997788.
- Alhandod TA, Rabbani SI, Almuqbil M, Alshehri S, Hussain SA, Alomar NF, Mir MA, Asdaq SMB. A Systematic Review on the Safety and Efficacy of COVID-19 Vaccines Approved in Saudi Arabia. Vaccines (Basel). 2023 Jan 28;11(2):281. doi: 10.3390/vaccines11020281. PMID: 36851158; PMCID: PMC9962734.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
