Journal of Vaccines and Immunology
Co-Administration of PPV23 and Influenza Vaccines in England and Wales: A Study Based on the Royal College of General Practitioners Sentinel Surveillance Network
Carl Selya-Hammer1*, Douglas Fleming2, Yiling Jiang1, Hayley Durnall2, Sam Keeping3 and Stuart Carroll3
2Royal College of General Practitioners, 30 Euston Square, London, NW1 2FB, UK
3Sanofi Pasteur MSD, Mallards Reach, Bridge Avenue, Maidenhead, SL6 1QP, UK
Cite this as
Selya-Hammer C, Fleming D, Jiang Y, Durnall H, Keeping S, et al. (2015) Co-Administration of PPV23 and Influenza Vaccines in England and Wales: A Study Based on the Royal College of General Practitioners Sentinel Surveillance Network. J Vaccines Immun 1(1): 043-049. DOI: 10.17352/jvi.000010Background: Pneumococcal disease is an infection caused by a bacterium called Streptococcus pneumonia which can lead to life-threatening invasive pneumococcal diseases. In the UK, pneumococcal vaccination is targeted at those most at risk of serious disease: infants, older people and those with risk factors. It has been proposed that PPV23 be co-administered with influenza vaccine during seasonal vaccination to maximize uptake. This study aimed to estimate 1) the UK prevalence of pneumococcal risk co-morbidities 2) corresponding pneumococcal vaccine administration rates 3) rates of vaccination co-administration and 4) frequency of pneumococcal revaccination.
Methods: To gather evidence on current vaccination practice in the UK and to quantify the rates of co-administration of influenza and pneumococcal vaccination, data was collected from general practices in the Royal College of General Practitioners sentinel surveillance network. To estimate the frequency of pneumococcal revaccination, the records of all persons vaccinated from 2010-2012 were examined and persons were counted according to the number of prior vaccine doses received since 2004.
Results: The prevalence rate for COPD was highest amongst the risk groups at 83.56 per 1,000 in 2010 and 79.33 in 2012. The vaccination rate was 6 per 1,000 among risk groups. Patients aged between 65 and 74 years showed the highest rate of vaccination (35 per 1,000), and rates were highest in the immun ocompromised and leukemia sufferers. Co-administration of pneumococcal and influenza vaccines increased sharply from 47% in 2011 to 61% in 2012. Among patients vaccinated against pneumococcal diseases, the proportion who had previously received a pneumococcal vaccination increased from 3 to 9 per 1,000 vaccinations from 2010 to 2012.
Conclusion: This study clarifies the current state of pneumococcal vaccination in England and Wales and highlights the need for vaccination coverage rates to be improved in order to prevent more cases of pneumococcal diseases.
Introduction
Pneumococcal disease is an infection caused by a bacterium called Streptococcus pneumoniae (pneumococcus) [1], which can lead to life-threatening invasive diseases such as bacterial pneumonia, meningitis and bacteremia, as well as non-invasive diseases [2-4]. Every year, S. pneumoniae infections account for approximately 1.6 million deaths worldwide and the incidence of invasive pneumococcal diseases (IPD) reaches 15 per 100,000 person-years [1]. In England and Wales, a recent study carried out by Public Health England (PHE; formerly Health Protection Agency [HPA]) reported on average 8,835 IPD cases annually [5]. S. pneumoniae infection secondary to influenza has been observed and highlighted in past influenza epidemics [6]. An influenza infection places individuals at risk of developing bacterial infections, a major complication of influenza which the highest risk in the winter months [7]. Coinfection with S. pneumoniae is considered as a predictor of severe outcome and a major cause of death [6-11].
Vaccination is a proven public health strategy one can implement to reduce the burden of IPD [3,12]. In the UK, pneumococcal vaccination is targeted at those most at risk of serious disease: infants, the elderly (≥65 years) and those with clinical risk factors. Age-based recommendations state that children should receive the conjugate pneumococcal vaccine (PCV) at 2 months, 4 months and between 12 – 13 months, while older people and at-risk patients receive pneumococcal polysaccharide vaccine (PPV23). At-risk patients, both children and adults, receive differing recommendations dependent on their clinical profiles. The clinical risk groups currently recommended to receive pneumococcal vaccination are presented in Table 1, and revaccination is recommended every 5 years for PPV23 for some groups in the high-risk population [13]. A select number of immunosuppressed patients are also recommended to receive a single dose of PPV23 followed by a single dose of PCV [16,17].
Given the similarity between their target populations, it has been proposed to co-administer PPV23 with influenza vaccine during the seasonal vaccination program to maximize uptake of the former [6]. Furthermore, evidence suggests clinical benefits exist from concomitantly administered vaccine in terms of an additive preventive effect [2,3], a strategy also recommended by PHE.
It is unclear how many pneumococcal vaccines have been co-administered with the influenza vaccine given PHE’s advocacy, nor has the revaccination rate been studied. This lack of data may create difficulties in the assessment of vaccination strategies. To address this gap, a retrospective database analysis was conducted based on the Royal College of General Practitioners (RCGP) sentinel surveillance network. The study’s main objectives were to estimate:
➢ The prevalence in the UK of persons with the various risk co-morbidities for whom pneumococcal vaccine is recommended
➢ The corresponding pneumococcal vaccine administration rates
➢ The rates of co-administration of the influenza and pneumococcal vaccines for the combined patient group
➢ The frequency of pneumococcal revaccination by age group
Material and Methods
Data was collected from general practices in the RCGP sentinel surveillance network, which has been shown to be representative of the national population of England and Wales by age, gender and social deprivation. The practices supplying patient records are broadly representative of England and Wales in terms of practice size, age group and practice prescribing patterns [18]. Rates of influenza vaccine uptake have been shown to be similar to the national equivalent uptake rate [19]. The RCGP database is prepared from a direct extract of electronic patient records generated as part of routine patient management. Relevant diagnostic and intervention information is recorded at the time of consultation and stored as Read codes which are mapped to ICD-9 codes for analysis.
The RCGP database was reviewed based on Read codes which 1) described pneumococcal vaccination or vaccine administration; 2) defined risk conditions for influenza vaccination uptake, published and known as PRIMIS code groups [20]; 3) were selected as applicable to risk conditions, even though the conditions were outside the influenza vaccination recommendations; and 4) described malignant disease (leukemia and related conditions and all other malignant neoplasms). Persons in these groups are very likely to be immune suppressed at least in the active treatment periods.
The first stage of the analysis aimed to estimate the prevalence of persons with risk co-morbidities for consideration of pneumococcal vaccination. Risk groups included malignant neoplasms, asplenia, hemolytic anemia, cystic fibrosis and combined pneumococcal specific risk groups. The prevalence rates of those at risk were calculated in 2010-2012 prior to September 1 in each year.
We then sought to estimate pneumococcal vaccine administration rates in the at-risk populations, defined by the presence of risk co-morbidities reported at least once from 2010-2012. Pneumococcal vaccine codes corresponded either to vaccine or vaccine administration. Vaccine uptake rates of the last 5 years were measured in total population age groups and in persons with risk co-morbidities.
The third stage aimed to quantify the rates of co-administration of influenza and pneumococcal vaccines to inform future analyses of vaccination costs. Vaccination data in each of three years were examined and results were consolidated into three age groups (0-14, 15-64 and 65+).
Lastly we estimated the frequency of pneumococcal revaccination by age. Records of all persons vaccinated in 2010-2012 were examined and persons were counted according to the number of prior vaccine doses received since 2004. The analysis was not conducted separately by risk group (which were not fixed over the study duration). Those receiving the pneumococcal vaccine from 2004-2012 were grouped by the number of vaccines received. All patients who had more than one vaccine recorded were examined separately and then by age group, to study the interval between vaccinations, with the analysis limited to the interval between the first and second vaccinations.
Results
Population eligible to receive pneumococcal vaccination
The RCGP database in 2010 included a total of 1.08 million patients, 1.02 million patients in 2011 and 603,000 in 2012. Estimates of the prevalence of persons with risk co-morbidities appropriate for consideration of pneumococcal vaccination are reported in Table 2 which presents the three-year estimates for prevalence per 1,000 population by age and risk group from 2010 to 2012. The prevalence rate for chronic obstructive pulmonary disease (COPD) was highest at 83.56 per 1,000 in 2010, 82.77 in 2011, 79.33 in 2012, followed by diabetes and chronic heart disease (CHD) which showed similar, if slightly increasing, prevalence rates in all three years.
Pneumococcal vaccine administration rates
Estimates of the pneumococcal vaccine administration rates are presented in Table 3 as the rate per 1,000 population by age group for the general population and by risk group. Using records from 114 practices in 2010, 6,998 (6 per 1,000) were found to have received the vaccine. Patients aged between 65 and 74 years showed the highest rate of vaccination (35 per 1,000), and rates did not differ significantly between males and females. Currently about 8 per 1,000 persons older than 2 years of age receive the pneumococcal vaccine annually.
Rates by risk group were calculated from the previous estimates of the prevalence of patients with risk-comorbidities. For example, 90,111 persons were identified with COPD (prevalence of 88 per 1,000), of whom approximately 1% received the vaccine in 2010. With the exception of the highly specific pneumococcal risk group (cochlear implant and cerebrospinal fluid leak risk), all co-morbidity groups are exclusive, but persons may have several co-morbidities. The risk groups corresponding to chronic kidney disease and the combined pneumococcal specific risk group had rates in 2012 that were two standard errors higher than the 2010 rate (representing a doubling of the vaccination rate from 2010 to 2012).
The number of individuals classified in the risk groups receiving pneumococcal vaccine totaled approximately 5,000 compared with 6,998 total vaccinated patients in the study year. This difference arises because many persons over 65 years were vaccinated without being classified as having a chronic condition. Current rates of vaccination were highest in the immunocompromised and leukemia sufferers.
Co-administration of pneumococcal and influenza vaccines
Estimates of the rates of co-administration of influenza and pneumococcal vaccines by age and gender are presented in Table 4. The number of influenza and pneumococcal vaccines was significantly higher in 2010 than in the two subsequent years as reported in Table 5, owing to the high number of persons receiving pandemic vaccinations. Results were therefore not aggregated for the three years to avoid confounding the data on co-administration. Vaccination data from the years 2011 and 2012 show a total of 148,933 males and 190,656 females vaccinated against flu, some of whom received repeat vaccinations in more than one season. The 5,841 male and 6,366 female pneumococcal vaccinations however are unlikely to include any double counting of persons (excepting those few persons given additional vaccine doses in separate years studied).
The data revealed several gender differences in vaccination rates. Influenza vaccination in children 14 years of age and younger recorded slightly more males than females, whilst in older age groups there were more females vaccinated. In light of the higher prevalence of asthma in male children, higher rates of vaccination against flu are to be expected. The higher rate of vaccination in females aged 15-64 can be explained by the vaccination of pregnant women. The higher number of influenza vaccination among females in the 65 years and older age group results from the increased proportion of females within this age group of the general population.
As shown in Table 4, the proportion of patients receiving co-administration of the pneumococcal and influenza vaccines increased sharply from 47% in 2011 to 61% in 2012 (p=<0.01). Combined analysis (2011 and 2012) showed 55% of male and 52% of female recipients who received the pneumococcal vaccine also received the influenza vaccine on the same day. This figure was 35% in the 15 to 64 years old group and 54% in the 65 years and older group.
Revaccination
Following the analysis of records of all persons vaccinated from 2010-2012 and the number of prior vaccine doses received since 2004, estimates of the frequency of pneumococcal revaccination were obtained by age group (Table 6). Among patients vaccinated against pneumococcal diseases, the proportion previously receiving at least one pneumococcal vaccination increased from 3 to 9 per 1,000 vaccinations in 2012. These figures are unlikely to include any double counting as the numerator accounts only for those patients vaccinated in a specific year; any duplication would be restricted to those patients receiving two doses within a given year.
The proportion of persons having received more than one pneumococcal vaccine increased year by year from 2010 to 2012, although remains below 1% of the total vaccinated population. Using data collected on all persons with more than one vaccination, an analysis was then conducted on the interval between the first and second vaccinations according to age, presented in Table 7. There were 68 persons receiving both vaccines on the same day, the majority (58) of whom were children less than 2 years of age. Instances where a subsequent primary dose was coded to the first date of administration may explain these occurrences, although it may also be due to other incorrect data entry in some practices. Two-thirds of second doses (348 doses, 67% of total) were given more than 5 years following the first vaccination and only 35 (6.7%) received a second dose within 1-364 days following the first vaccination.
Discussion
Approximately 8 per 1,000 population were given the pneumococcal vaccine each year, whereby population is defined as the RCGP database population. The highest rates were seen in the 65-74 age group, in which approximately 40 per 1,000 individuals were vaccinated, and among risk groups were highest in the immunocompromised and leukemia sufferers.
Rates of co-administration of the influenza and pneumococcal vaccines showed that in adults, just over 50% of pneumococcal vaccines were co-administered with the influenza vaccine, with 38% in vaccinated children. There were small differences in this rate between sex and age groups attributable to the demographics of the population, prevalence of COPD (higher in males) and routine influenza vaccination of pregnant women.
Two-thirds of persons receiving repeat vaccination received this at the recommended interval of 5 years minimum, but for a significant number (greater than 30%), there may be revaccination within 5 years.
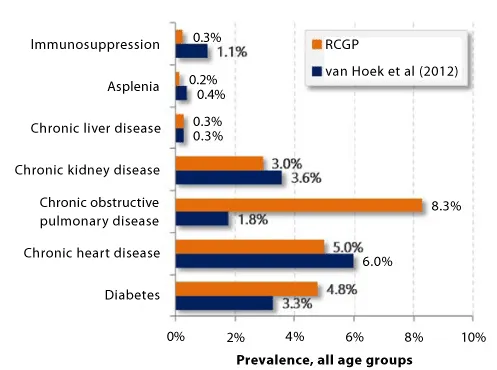
A previous study by Van Hoek et al. [21], used a Department of Health survey on the uptake of 23-valent pneumococcal polysaccharide vaccines using data extracted from 55% of the general practices in England to estimate the prevalence of clinical risk factors in the general population. Rather than using 3-year prevalence rates as in the current study, the authors estimated prevalence rates based on 2008-2009 data alone.
The 3-year prevalence rates of clinical risk factors presented in the current study could be expected to be higher, with discrepancies due in part to certain diseases’ changing prevalence rates and data search methodologies of the two studies. Van Hoek et al. used data from ImmForm to record data on uptake of immunization programs and incidence of flu-like illness, covering 60% of the population without assumptions on whether the patient was currently impacted by the disease coded in her records. Diseases such as asplenia may be recorded while not prompting a consultation for most of the patient’s life. Similarly, immune suppression is detected from a combination of disease and prescription codes and attempts to detect contemporary immune suppression rather than any period of immune suppression. The database used in the current study did not include these corresponding prescription codes which may have contributed to lower prevalence rates presented in Figure 1.
The current study’s higher diabetes prevalence is linked to the inclusions of all diabetics (whether on therapy or diet management) and to its multiyear time horizon. The inclusion of all diabetics reflects an update to influenza vaccination policy which now includes all diabetics. Incidentally pneumococcal vaccination policy has not been updated to include all diabetics [22], and in our view an alignment of these vaccination policies would be greatly beneficial.
This is the first study we are aware of to investigate the co-administration and revaccination rates of pneumococcal vaccination. Our data may assist decision-makers in developing a strategy to improve the coverage of pneumococcal vaccine in the older population so that more disease cases associated with S. pneumoniae infection can be avoided. This is especially true in England and Wales as the study population is representative of the population in terms of age, gender, social deprivation and practice size [18] and the results are in line with an earlier study by the PHE [21].
Several limitations must also be acknowledged. The morbidity of the study population has not been compared to the national population, though this is currently being investigated. Secondly, the analysis of time to revaccination was based on a relatively small sample size considering the recommendation was for a subset of the target population. More data are needed to provide robust estimates. This study included patients with malignant disease as separate risk group, although malignant disease as such is not included in the Green book. Many would argue that these diseases result in impaired immune response whether the patient is receiving an immune suppressant therapy or not. Our results provide additional information on this specific risk group should policy change.
The study provides a clearer picture of the current situation of pneumococcal vaccination in England and Wales, and has presented a vaccination rate that unfortunately remains low. In the at-risk population, the three-year cumulative vaccination rate ranged between 50 and 150 per 1,000 person-years, significantly lower than the uptake rates for influenza vaccination in winter 2012/13, reported to be 73.4% for those aged 65 years and over and 51.3% for those aged six months to under 65 years in one or more clinical at-risk groups [23]. Strategies to improve vaccination coverage rates in the eligible population will lead to the prevention of avoidable disease and should be seen as a priority.
Routine vaccine co-administration could provide such an avenue to higher coverage rates. Assuming administration is carried out by a band 7 advanced nurse in the course of a 10 minute appointment [24], an administration session can cost £15.17, which is almost equivalent to the cost of two pneumococcal vaccines (£8.32; Pneumovax® II, Sanofi Pasteur MSD). More than half of the patients vaccinated against pneumococcal disease within this study received the vaccine co-administered with the influenza vaccine. The current practice of co-administration improves the cost-effectiveness of both programs as it reduces the combined cost of administering the two vaccines by 17.3% compared to separate administration. Improving the co-administration rate would reduce opportunity costs while also leading to higher vaccination coverage rates for the pneumococcal vaccination program. Data from a recent study [25], showed that when co-administration is considered, incremental cost-effectiveness ratio decreased from £14,895 to £8,413 per quality-adjusted life year gained. Future economic evaluations would benefit from taking co-administration into account to ensure the true cost the vaccination program can be accurately estimated.
Conclusion
From a sample of over one million patients we presented the prevalence rates for risk groups recommended to receive the pneumococcal vaccine by age group and gender for the three years from 2010 to 2012. In 2010, these prevalence rates ranged from approximately 0.001% in patients with cystic fibrosis to 8.36% for those with COPD.
Rates of pneumococcal vaccine administration were found to be approximately 8 per 1,000 study population, with the highest rates seen in age group 65-74 years and in immunocompromised patients, leukemia sufferers, people with chronic kidney disease and those with cystic fibrosis. Over 50% of pneumococcal vaccines were estimated to be administered at the same time as influenza vaccine, and two-thirds of persons receiving repeat vaccination received it at the recommended interval of 5 years minimum, with some receiving early revaccination.
This study provides a clearer picture of the current situation of pneumococcal vaccination in England and Wales and highlights the need for vaccination coverage rates to be improved in order to prevent more cases of pneumococcal diseases. One possibility includes the use of co-administration of pneumococcal and influenza vaccines as useful tool to support the implementation of the pneumococcal vaccination program.
- Musher DM (2010) Streptococcus pneumoniae. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier 2623-2642.
- Lynch JP 3rd, Zhanel GG (2009) Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med 30:189-209.
- Prato R, Tafuri S, Fortunato F, Martinelli D (2010) Why it is still important that countries know the burden of pneumococcal disease. Hum Vaccin 6: 918-921.
- Fedson DS, Nicolas-Spony L, Klemets P, van der Linden M, Marques A, et al. (2011) Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines 10: 1143-1167.
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC (2011) Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 11: 760-768.
- Gilchrist SA, Nanni A, Levine O (2012) Benefits and effectiveness of administering pneumococcal polysaccharide vaccine with seasonal influenza vaccine: an approach for policymakers. Am J Public Health 102: 596-605.
- (2014) European Centre for Disease Prevention. Fact sheet for the general public.
- Tashiro M, Ciborowski P, Reinacher M, Pulverer G, Klenk HD, et al. (1987) Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology 157: 421-430.
- McCullers JA, Bartmess KC (2003) Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis 15; 187: 1000-1009.
- McCullers JA (2006) Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 19: 571-582.
- Diavatopoulos DA, Short KR, Price JT, Wilksch JJ, Brown LE, et al. (2010) Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J 24: 1789-1798.
- Miller, Elizabeth, et al. (2011) Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 11: 760-768.
- (2013) Health Protection Agency. Pneumococcal vaccination recommendations.
- (2012) Pneumococcal. In: Salisbury D, Ramsay M, Noakes K, editors. Immunisation against infectious disease (the Green Book). 4th ed. London: The Stationery 295-313.
- (2013) Joint Committee on Vaccination and Immunisation. JCVI statement on the wider use of pneumococcal conjugate vaccines in the UK.
- Nichol KL (1999) The additive benefits of influenza and pneumococcal vaccinations during influenza seasons among elderly persons with chronic lung disease. Vaccine 17: S91-S93.
- Christenson B, Hedlund J, Lundbergh P, Ortqvist A (2004) Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur Respir J 23: 363-368.
- Fleming DM, Miles J (2010) The representativeness of sentinel practice networks. J Public Health (Oxf) 32: 90-96.
- (2013) Imm Form.
- Gates P, Noakes K, Begum F, Pebody R, Salisbury D (2009) Collection of routine national seasonal influenza vaccine coverage data from GP practices in England using a web-based collection system. Vaccine 27: 6669-6677.
- van Hoek AJ, Andrews N, Waight PA, Stowe J, Gates P, et al. (2012) The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect 65: 17-24.
- (2013) Immunisation against infectious disease. London, UK: Public Health England
- (2013) Public Health England. Influenza vaccine uptake amongst GP patient groups in England for winter season 2012 to 2013
- Baguelin M, Jit M, Miller E, Edmunds WJ (2012) Health and economic impact of the seasonal influenza vaccination programme in England. Vaccine 30: 3459-3462.
- Jiang Y, Gauthier A, Keeping S, Carroll S (2014) Cost-effectiveness of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Rev Pharmacoecon Outcomes Res 14: 913-927.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
