Global Journal of Infectious Diseases and Clinical Research
Serial measurements of SIRS criteria to identify unique phenotypes of sepsis: A Microbiologic Approach
Harman S Gill1-3*, Phuong H Nguyen1, Jada M English1, Kayla A Fay4, Elisha Fleig Yin MPAS5, Jaskirat Kaur Gill6, Todd D Morrell1,2
2Department of Medicine, Section of Pulmonary & Critical Care Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA
3Department of Emergency Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA
4Department of Surgery, Section of Thoracic Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA
5Respiratory Institute, Cleveland Clinic, CLEVELAND, OH, USA
6Department of Emergency Medicine and Institute of Critical Care Medicine, Mount Sinai Morningside, New York, NY, USA
Cite this as
Gill HS, Nguyen PH, English JM, Fay KA, Yin MPAS EF, et al. (2023) Serial measurements of SIRS criteria to identify unique phenotypes of sepsis: A Microbiologic Approach. Glob J Infect Dis Clin Res 9(1): 016-024. DOI: 10.17352/2455-5363.000057Copyright
© 2023 Gill HS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: The utility of serial scoring systems in identifying distinct sepsis phenotypes remains unknown.
Methods: Eligible adults were classified into culture-positive (Cx+) and culture-negative (Cx-) groups alongside pre-defined culture subgroups. Average SIRS & SEP (novel scoring system) scores were calculated at t = 0 and hours 3,6,12 & 24 before and after t = 0. The primary outcome was a difference in SIRS/SEP scores amongst those that were Cx+ or Cx- at any time point. Secondary outcomes were comparing total and component SIRS/SEP scores in microbiologic subgroups over serial time points.
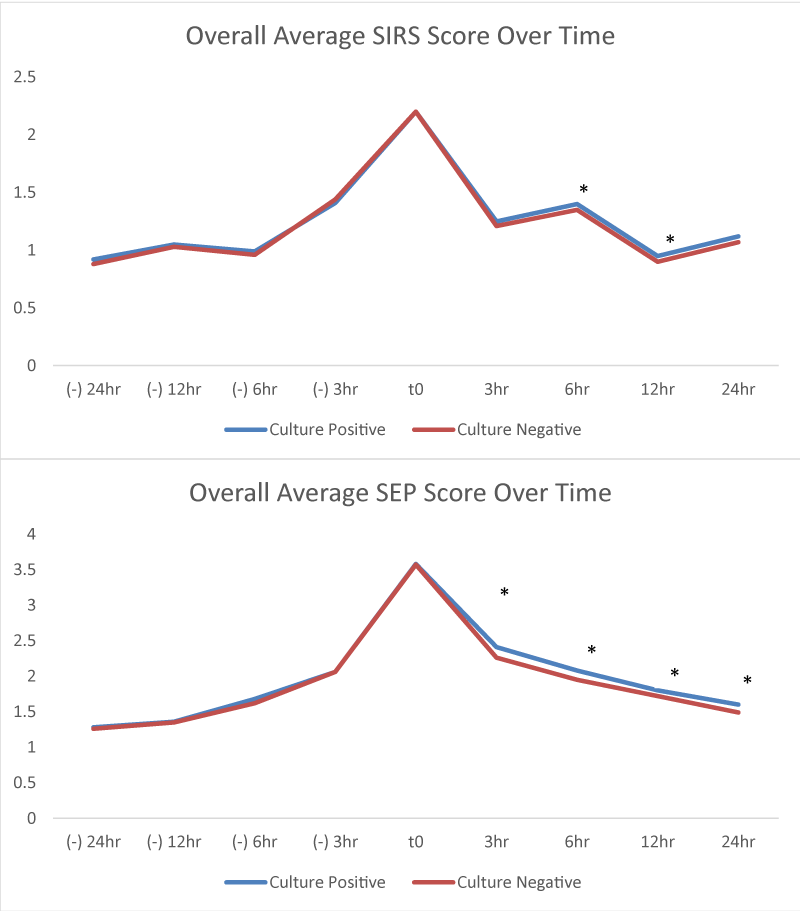
Results: 4,701 Cx+ and 3254 Cx- patients met eligibility criteria. Statistically significant differences were seen in the average SIRS score between Cx + and Cx- groups at hours six (Cx+ 1.40+1.04 vs Cx- 1.35+1.01) & 12 (Cx+ 0.95+0.95 vs Cx- 0.90+0.90) after t = 0. The hematologic, urologic, and neurologic subgroups had significant differences at numerous time points before and after T = 0. Similar findings were observed with the SEP scores. Cx+ and Cx- groups (including subgroups) consistently doubled both SIRS/SEP scores before t = 0 with an eventual return to baseline values after T = 0 but at different gradients.
Conclusion: Significant differences in SIRS/SEP scores were seen in Cx+ & Cx- patients at sequential time points. This microbiologic approach in homogenous culture cohorts has the potential to identify distinct phenotypes of sepsis efficiently and practically. Consistent increases in SIRS/SEP scores before t = 0 and sequential decreases after t = 0 may allow for early detection, intervention, and provision for real-time monitoring of therapeutic responses in patients with concerns for sepsis.
Introduction
Sepsis and septic shock continue to be a significant cause of in-hospital mortality [1,2]. The lack of a clear gold standard for the diagnosis of sepsis [3,4], nor a clearly identifiable syndrome initiation time, T = 0, remain major barriers to successful diagnosis and create delays in treatment initiation [5,6]. Over time, different diagnostic algorithms have been devised but every system has had its limitations [7-10]. Furthermore, most scoring systems look only at diagnostic variables at a single time point and not as a continuum seen in the clinical syndrome that typifies sepsis. Recent studies indicate that serial clinical assessments not only have clear utility in prognostication [11,12] but also higher diagnostic accuracy over single-time point evaluations [13-17].
Seymour, et al. [18] have also recently described distinct clinical phenotypes of sepsis with clear implications for timely diagnosis and management. While their work was the first of its kind, the use of sophisticated immunologic and inflammatory biomarkers could make the routine bedside utilization of their approach challenging.
Yet, many data points, including microbiologic data are readily available in the routine evaluation of patients with concerns for sepsis. In querying the relevant literature, it seems like an approach centered on positive or negative microbiology in those that triggered clinical concerns for sepsis to identify distinct phenotypes of sepsis has not been investigated. Thus, the primary objective of this study was to look at common scoring systems for suspected or confirmed sepsis and identify differences in how culture-positive and negative sepsis cohorts behave at the time clinical concerns for sepsis are first identified and how those cohorts evolve over time.
Methods
Study population
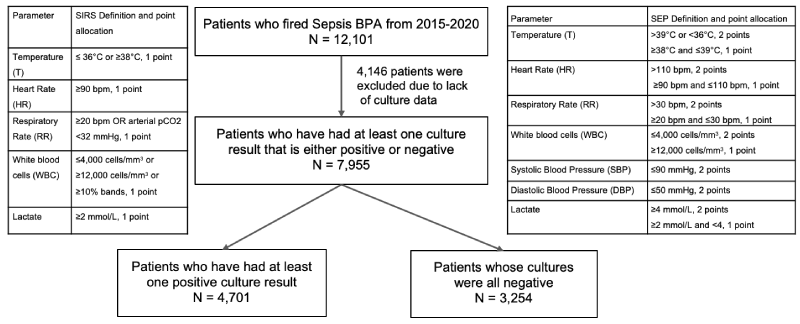
Patients aged≥18 who fired our sepsis best practice advisory (BPA) between 2015-20 were included in this retrospective analysis. Our sepsis BPA used the Systemic Inflammatory Response Syndrome (SIRS) [19] scoring system & would fire when a patient had a score of 2 or greater (Figure 1). Patients who did not have available culture data had missing data fields or those who had a sepsis BPA fire while in the intensive care unit were excluded. Cultures consistent with contamination (per Infectious Disease Society of America - IDSA guidelines) were also excluded.
Data collection & objectives
After inclusion and exclusion criteria were assessed, patients were separated by culture positivity. Patients were designated as being culture positive (Cx+) if any microbiologic specimen drawn after the sepsis BPA had fired was consistent with a true positive. Similarly, a patient was designated as culture negative (Cx–) if their culture assays had no growth when considered final by the microbiology lab OR had no microbiologic specimen consistent with a true positive result per IDSA guidelines mentioned above. The culture positive and negative groups were further divided into organ system cohorts (respiratory - RESP, musculoskeletal/skin - MSK, gastrointestinal - GI, genitourinary - URO, hematologic - heme, neurologic - neuro, Miscellaneous – MISC, and “other”). The hematologic group included patients who had bacteremia. The miscellaneous category included infected hardware such as joint prostheses or pacemaker leads. The other category reflected patients with fungemia and/or viremia.
Collected data points included microbiologic data as stated above, vital signs [Heart Rate (HR), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Temperature (T), Respiratory Rate (RR)], and lab values [white blood cell count (WBC), lactate]. To further amplify vital signs and lab differences, a novel scoring system called the SEP was created. The rubric for this SEP score and point allocation is also described in Figure 1. Average hospital Length of Stay (LOS), days from admission to BPA fire, and days from BPA fire to discharge were also calculated for all subgroups.
Once all patients were divided into Cx+ & Cx- cohorts, the average SIRS & SEP scores were calculated at the time the BPA fired (defined as T = 0). Average SIRS & SEP scores were subsequently calculated in each individual culture subgroup. This analysis of average SIRS & SEP score that was initially done at the time of the BPA fire was then repeated at 24, 12, 6, and 3 hours before the BPA fired; and 3, 6, 12, and 24 hours after the BPA fired for all cohorts. Lastly and only within each culture subtype, the components of HR, SBP, DBP, T, RR, lactate, and WBC were analyzed for their progression over the various time points.
The primary objective of the study was to identify differences in SIRS/SEP scores amongst those that were Cx+ or Cx- at any time point. Secondary outcomes were comparing total and component SIRS/SEP scores over consecutive time points. The null hypothesis was that there would be no significant differences in average SIRS/SEP scores between the Cx + & Cx – cohorts at the varying time points and in different culture subgroups.
Statistics
Average in-hospital mortality as well as in-hospital mortality plus discharge to hospice for each subgroup was calculated and compared using a Chi-squared test. Average SIRS/SEP scores, as well as component vital signs and lab values, were calculated and compared using a two-sample t-test for all time points. A p - value <0.05 was deemed statistically significant. Analysis was performed using Stata/IC (College Station, TX: StataCorp LLC). This study was approved by the Dartmouth Hitchcock Institutional Review Board. The IRB and ethics board approval number is STUDY02001065. All data were obtained under an informed consent waiver and were de-identified.
Results
Of the 12,101 patients who met inclusion criteria, 4,146 patients were excluded due to lack of culture data, with a total of 7,955 patients included in the final analysis (Figure 1 & Appendix A). In-hospital mortality (IHM) and IHM alongside new discharge to hospice as a composite and for all culture subgroups are shown in Table 1 and Appendix B, C. Length of Stay (LOS), days from admission to BPA fire, and days from BPA fire to discharge can be found in Appendix D, E, and F respectively. Average in-hospital mortality, LOS, new discharge to hospice, and days from BPA fire to discharge values were all higher & statistically significant for Cx + patients in all composite and many subgroup analyses.
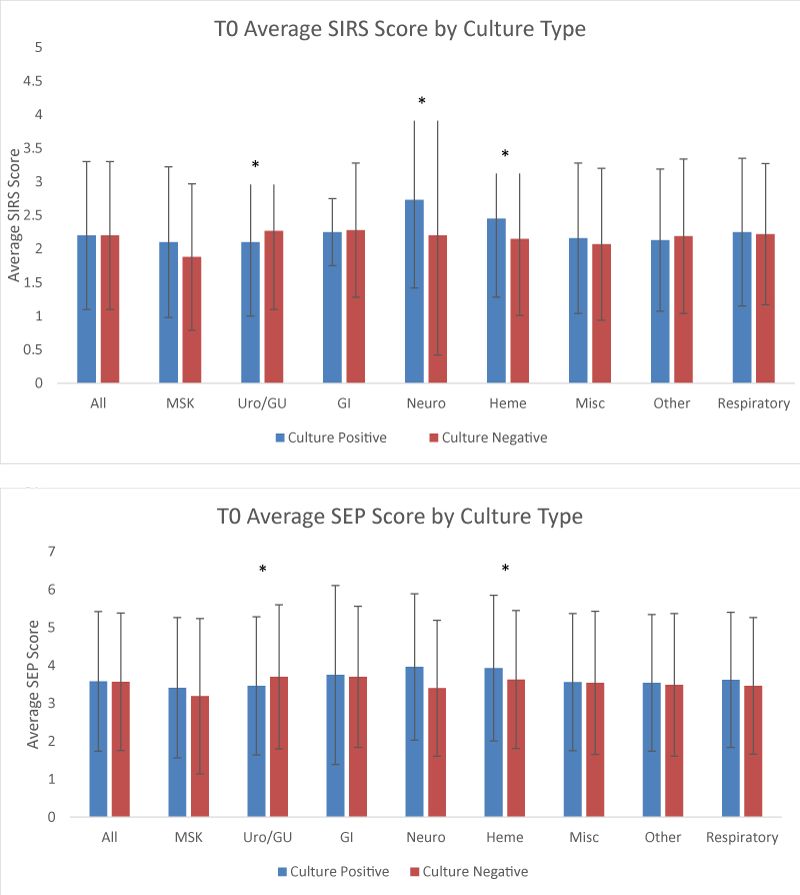
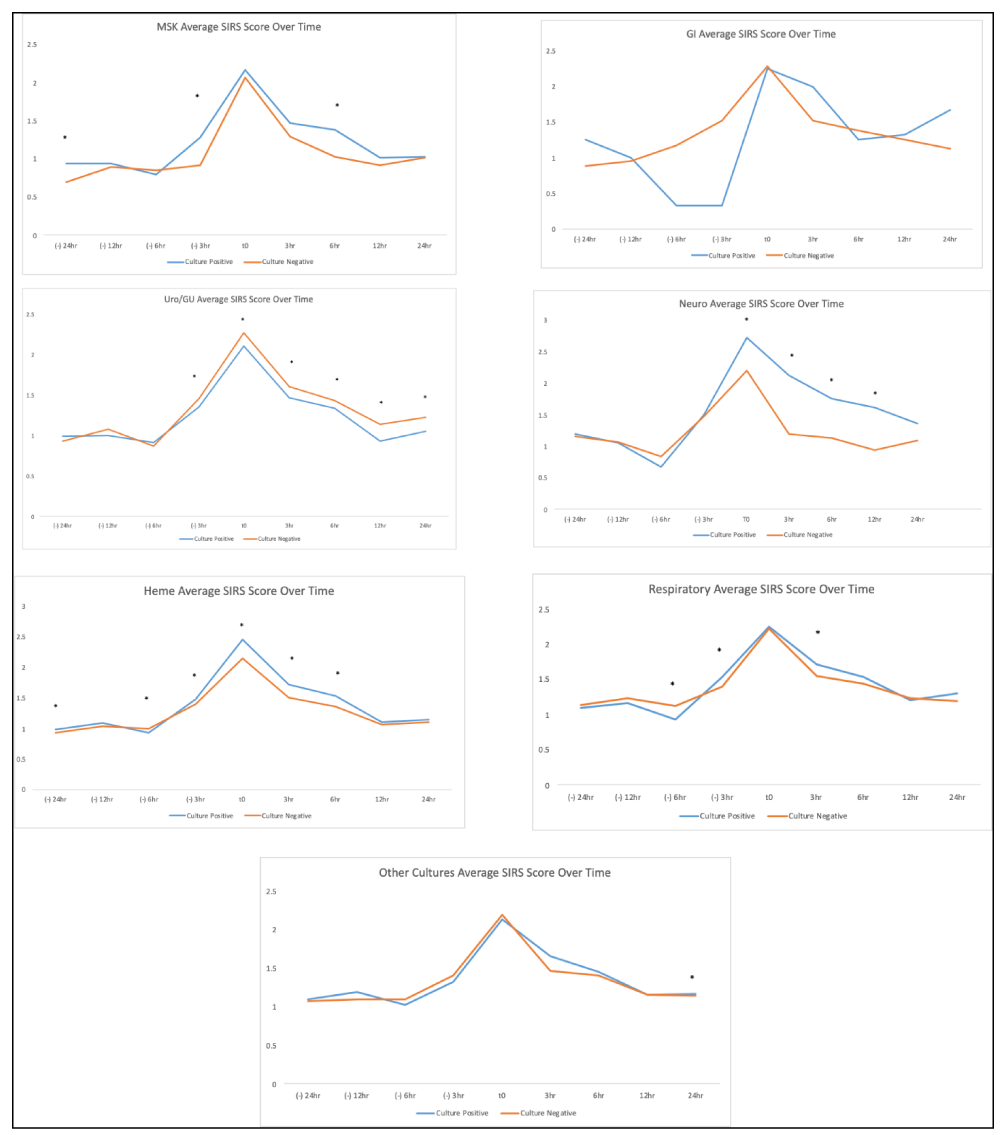
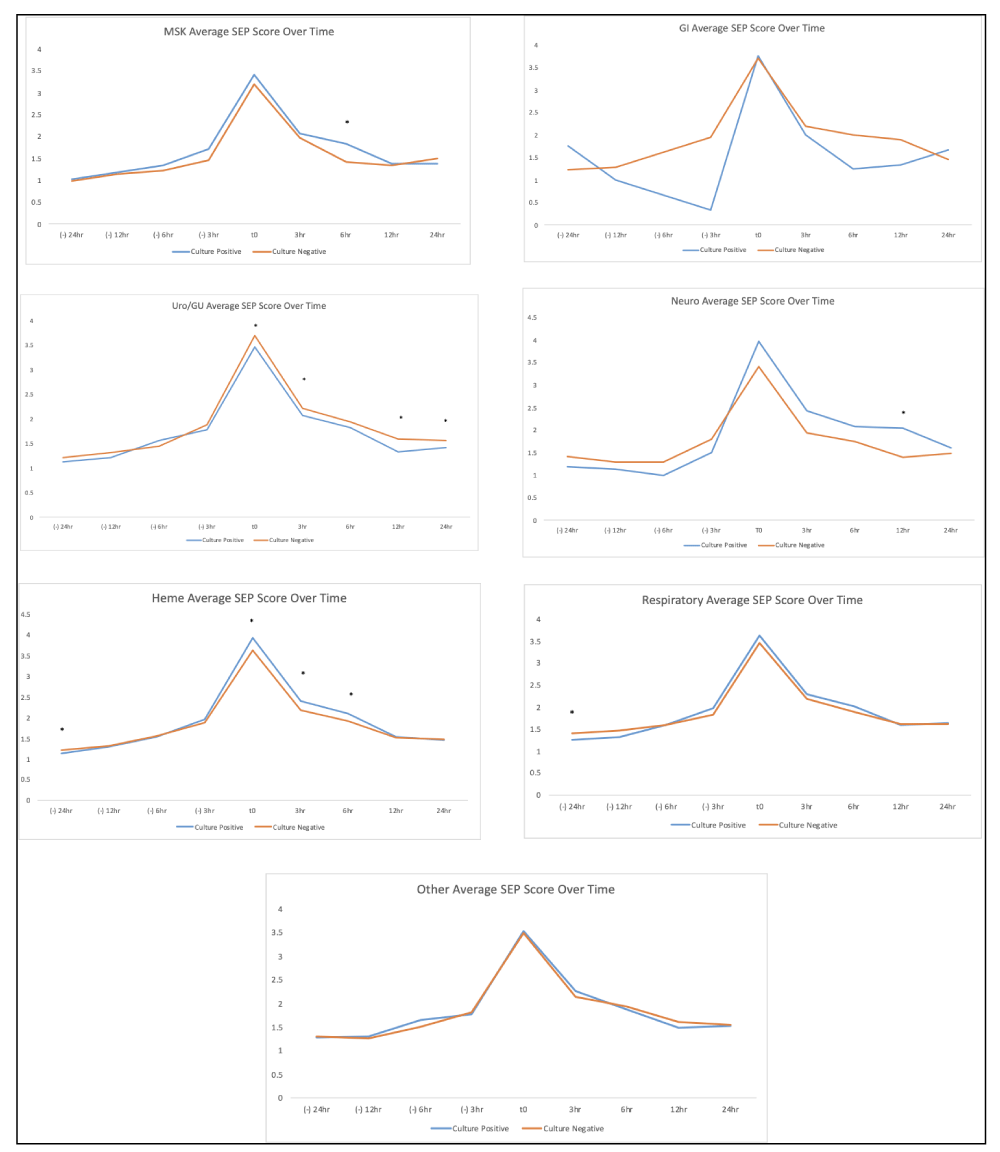
The average SIRS and SEP scores at the time of the BPA fire were 2.19 + 1.12 and 3.57 + 1.85 respectively for all Cx + patients, and 2.22 + 1.14 and 3.57 + 1.81 respectively for all Cx - patients. Average SIRS/SEP scores as a composite for all subgroups at the time of BPA fire (T = 0) are shown in Figure 2. Average SIRS/SEP scores over time for all Cx+/Cx - patients are seen in Figure 3. SIRS and SEP scores over time based on the system of infection are found in Figure 4 and Figure 5 respectively. Individual components (such as RR, T, etc.) that formed the SIRS/SEP score in each culture subtype were also analyzed for progression over time and results are shown in Appendix G&H. When looking at heterogeneous composite groups (all Cx+ & Cx -), there were no statistically significant differences. Yet, many homogenous subgroups (heme, uro, neuro) and individual components were statistically significant in their differences between Cx + and Cx – cohorts at numerous and sequential time points.
Discussion
This single-center retrospective study looked at culture-positive (Cx+) and culture-negative (Cx -) patients with presumed sepsis to further identify and classify sepsis phenotypes. We used SIRS as well as a novel scoring system, SEP, in Cx+/Cx – patients in a serial fashion. We found a statistically significant difference in mortality, LOS, days from admission to BPA fire, and days from BPA fire to discharge between all Cx + and Cx - cohorts. Heterogeneous groups that combined all Cx + and all Cx - patients, showed no differences in their cumulative SIRS/SEP scores at T = 0. Yet, when more homogenous subgroups were analyzed at T = 0 and at serial time intervals before and after T = 0, statistically significant differences were observed in many groups and time points. All cohorts showed a clear window of bioreactivity and a window of therapeutic responsivity on either side of T = 0. Cx + subgroups had higher SIRS & SEP scores before and after T = 0 with some unique cohorts even reaching statistical significance for individual component variables such as respiratory rate and temperature. As far as we are aware, this is the first study comparing serial SIRS/SEP scores between Cx + and Cx - cohorts with a specific focus on addressing intrinsic heterogeneity via culture subtypes and individual contributing components of the SIRS/SEP scores.
Sepsis and septic shock remain a clinical syndrome that has no clear and unanimously agreed upon gold standard for its definition or exact time of onset [20-26]. Over the years, many revisions to diagnostic scoring systems and management guidelines have been made. Unfortunately, no one approach has been shown to have clear diagnostic or management superiority [27,28]. In addition, many of the individual portions of the initially protocolized algorithms of early goal-directed therapy have also been brought into question with regard to their practical utility and diagnostic accuracy [29-34]. In general, a shift in management strategies has occurred from using one-time, static parameters such as serum lactates and central venous oxygen saturations to serial, dynamic, and real-time parameters such as change in serum lactates (delta lactates) or procalcitonin values [35-45]. Our study looked to assess the potential role of measuring cumulative sepsis scoring systems such as SIRS in a serial fashion and assess its impact in homogenous patient populations such as refined microbiologic cohorts to see if there were any meaningful clinical trends for earlier clinical detection and ongoing resuscitation.
We found that in-hospital mortality, LOS, days from admission to BPA fire, and days from BPA fire to discharge values for composite analyses of all Cx + patients were significantly higher when compared to Cx - patients. This pattern repeated itself in homogenous subgroups such as respiratory, MSK & heme for both LOS and days from fire to discharge, with higher values for Cx + patients. Furthermore, component values such as average heart rates and serum lactates in our Cx + cohorts were comparable to those in the gamma and delta phenotypes (higher mortality, rates of shock, ICU admission, etc.) described by Seymour, et al. [18]. This pattern supports the hypothesis that despite the amount of heterogeneity, culture positivity could be an identifier of important phenotypes of patients with sepsis owing to major mortality, morbidity, and patient-centered outcome differences. Furthermore, this microbiologic information is routinely collected in all patients being evaluated for sepsis. Thus, this approach allows the exact clinical tenets of precise therapy and improved care described by Seymour et al to be more achievable, practical, and realistic.
No significant differences in average SIRS/SEP scores were noted when the combined groups of all Cx + or all Cx - patients were analyzed at T = 0. Yet, even at T = 0, when relatively homogenous patients were studied in refined cohorts (heme, neuro, Uro/GU), there were numerous statistically significant differences appreciated not only in the average SIRS/SEP scores but also in the individual components that formed the scores at T = 0. These findings in the refined culture subgroups were again noted over serial time points and further highlighted the importance of addressing heterogeneity in the contiguous clinical syndrome that is sepsis [18]. Culture subtypes that did not show statistically significant trends are likely secondary to the low power of those individual subgroups and likely suffer from major statistical fragility that should be addressed in larger multi-centered trials.
Another unique aspect of this trial is using serial SIRS scores to better understand the evolution of patients with sepsis over time in the construct of culture positivity or negativity. Recent studies have demonstrated increased sensitivity of using serial vital sign changes compared to one-time vital signs for identifying sepsis [13,14,46]. Yet, to the best of our knowledge, no studies have used such serial assessments in the clinical paradigm of positive v/s negative microbiology. This approach may allow the sensitivity/specificity inherent to a scoring system like SIRS to be compounded and potentially increase the predictive accuracy within a culture subtype to identify a more refined phenotype closer to true positive sepsis physiology. This is of particular importance as sepsis remains a clinical syndrome that lacks a true gold standard for diagnosis. While far from perfect and an individual patient’s physiologic derangements in our study may not be completely explained by sepsis alone, it stands within reason to consider that any patient who triggers two or more SIRS criteria AND has positive microbiology (as in our Cx+ cohorts) has some portion of their physiologic derangements attributable to true sepsis physiology.
All patients, regardless of microbiology, showed a clear window of bioreactivity, characterized by an increasing SIRS/SEP score leading up to T = 0, and a window of therapeutic responsivity characterized by a decreasing SIRS/SEP score after T = 0, albeit with different gradients. These windows were also seen in subgroups (heme, resp, neuro, uro/GU) and had significant differences in average SIRS/SEP scores between those that were Cx + and those that were Cx - at each individual and consecutive time points. These data suggest that these differences are less likely to be statistical anomalies and more likely due to underlying pathophysiological differences as they persist across different time points. These incremental increases in SIRS and SEP scores before T = 0 suggest a potential window for earlier detection and intervention of hitherto unidentified patients with sepsis even with conventional scoring systems like SIRS. Meanwhile, the eventual reduction in SIRS and SEP scores may also suggest a potential measure by which response to therapies can be assessed in real-time. For instance, sustained higher SIRS/SEP scores after T = 0 may indicate to the treating clinician that the patient may eventually have positive microbiology and important patient-centered outcome differences such as higher rates of mortality, LOS, and new discharge to hospice compared to those with more rapid decrease of their SIRS/SEP score over time in response to therapies. Furthermore, both before and after T = 0, a delta in a particular SIRS or SEP score may have more of a value than an absolute SIRS or SEP cutoff for predicting patients with not only sepsis physiology but also sepsis physiology with eventually positive microbiology. This trend was true in multiple culture subtypes in our study when using the SIRS or SEP score and even in specific components that formed these scores. While not the focus or within the scope of our study, the specific component data could inform future work focused on deriving and validating an organ system-specific sepsis prediction tool or scoring system, for instance, patients with potential bacteremia as depicted by the heme group in our study.
In this study, we also described a novel clinical score criteria named the SEP score. This score was designed to better account for the severity of disturbances (for example, difference in heart rate) and amplify differences in specific subgroups. Though this study was not designed to compare the SIRS and SEP criteria, the data in Figures 2,3 are suggestive that the SEP score has internal validity when compared to SIRS and is able to amplify scoring differences when compared to the SIRS score. Further studies should be done to evaluate the clinical utility of the SEP score.
Limitations
The study had several limitations. First, this was a single-center, retrospective study. Some of the subgroups were underpowered with small sample sizes, and further studies with larger sample sizes may differ significantly in results. It is worth mentioning that we were looking at patients with presumed sepsis, and some patients likely had sepsis-like physiology (sepsis mimics) or sepsis mixed with other physiologic states such as myocardial dysfunction. While this study did not account for this confounder, the lack of a clinical gold standard for the true definition of this syndrome may not make this a feasible goal.
Conclusion
This study showed significant differences in SIRS/SEP scores in patients with positive and negative microbiology at serial time points. This points to culture positivity and negativity being an effective paradigm in identifying distinct clinical phenotypes of sepsis. Important culture subgroups were identified and a clear increase in SIRS score (window of bioreactivity) before T = 0 and a subsequent decrease in scores after T = 0 (window of therapeutic responsivity) was also noted. These findings of using SIRS criteria in a serial fashion suggest potential utilities in early detection, intervention, and a real-time provision for monitoring response to therapeutics in each phenotype. Future research should continue to investigate readily available methods of classifying and identifying different septic phenotypes and their response to various treatments.
The entire team is deeply thankful and grateful to Dylan Calhoun who helped collate data from the hospital-based sepsis registry.
- Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013 May;41(5):1167-74. doi: 10.1097/CCM.0b013e31827c09f8. PMID: 23442987.
- Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014 Jan 1;5(1):4-11. doi: 10.4161/viru.27372. Epub 2013 Dec 11. PMID: 24335434; PMCID: PMC3916382.
- Kalantari A, Mallemat H, Weingart SD. Sepsis Definitions: The Search for Gold and What CMS Got Wrong. West J Emerg Med. 2017 Aug;18(5):951-956. doi: 10.5811/westjem.2017.4.32795. Epub 2017 Jul 10. PMID: 28874949; PMCID: PMC5576633.
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003 Apr;29(4):530-8. doi: 10.1007/s00134-003-1662-x. Epub 2003 Mar 28. PMID: 12664219.
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589-96. doi: 10.1097/01.CCM.0000217961.75225.E9. PMID: 16625125.
- Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med. 2017 Jun 8;376(23):2235-2244. doi: 10.1056/NEJMoa1703058. Epub 2017 May 21. PMID: 28528569; PMCID: PMC5538258.
- Tusgul S, Carron PN, Yersin B, Calandra T, Dami F. Low sensitivity of qSOFA, SIRS criteria and sepsis definition to identify infected patients at risk of complication in the prehospital setting and at the emergency department triage. Scand J Trauma Resusc Emerg Med. 2017 Nov 3;25(1):108. doi: 10.1186/s13049-017-0449-y. PMID: 29100549; PMCID: PMC5670696.
- Liao MM, Lezotte D, Lowenstein SR, Howard K, Finley Z, Feng Z, Byyny RL, Sankoff JD, Douglas IS, Haukoos JS. Sensitivity of systemic inflammatory response syndrome for critical illness among ED patients. Am J Emerg Med. 2014 Nov;32(11):1319-25. doi: 10.1016/j.ajem.2014.07.035. Epub 2014 Aug 8. PMID: 25205616; PMCID: PMC4254326.
- Wang C, Xu R, Zeng Y, Zhao Y, Hu X. A comparison of qSOFA, SIRS and NEWS in predicting the accuracy of mortality in patients with suspected sepsis: A meta-analysis. PLoS One. 2022 Apr 15;17(4):e0266755. doi: 10.1371/journal.pone.0266755. PMID: 35427367; PMCID: PMC9012380.
- Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015 Apr 23;372(17):1629-38. doi: 10.1056/NEJMoa1415236. Epub 2015 Mar 17. PMID: 25776936.
- Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001 Oct 10;286(14):1754-8. doi: 10.1001/jama.286.14.1754. PMID: 11594901.
- Chiew CJ, Wang H, Ong MEH, Wong TH, Koh ZX, Liu N, Feng M. Serial Heart Rate Variability Measures for Risk Prediction of Septic Patients in the Emergency Department. AMIA Annu Symp Proc. 2020 Mar 4;2019:285-294. PMID: 32308821; PMCID: PMC7153136.
- Quinten VM, van Meurs M, Olgers TJ, Vonk JM, Ligtenberg JJM, Ter Maaten JC. Repeated vital sign measurements in the emergency department predict patient deterioration within 72 hours: a prospective observational study. Scand J Trauma Resusc Emerg Med. 2018 Jul 13;26(1):57. doi: 10.1186/s13049-018-0525-y. PMID: 30005671; PMCID: PMC6045840.
- Shashikumar SP, Stanley MD, Sadiq I, Li Q, Holder A, Clifford GD, Nemati S. Early sepsis detection in critical care patients using multiscale blood pressure and heart rate dynamics. J Electrocardiol. 2017 Nov-Dec;50(6):739-743. doi: 10.1016/j.jelectrocard.2017.08.013. Epub 2017 Aug 16. PMID: 28916175; PMCID: PMC5696025.
- Pimentel MAF, Redfern OC, Hatch R, Young JD, Tarassenko L, Watkinson PJ. Trajectories of vital signs in patients with COVID-19. Resuscitation. 2020 Nov;156:99-106. doi: 10.1016/j.resuscitation.2020.09.002. Epub 2020 Sep 10. Erratum in: Resuscitation. 2021 May;162:91-92. PMID: 32918984; PMCID: PMC7481128.
- Samsudin MI, Liu N, Prabhakar SM, Chong SL, Kit Lye W, Koh ZX, Guo D, Rajesh R, Ho AFW, Ong MEH. A novel heart rate variability based risk prediction model for septic patients presenting to the emergency department. Medicine (Baltimore). 2018 Jun;97(23):e10866. doi: 10.1097/MD.0000000000010866. PMID: 29879021; PMCID: PMC5999455.
- Zhu Y, Chiu YD, Villar SS, Brand JW, Patteril MV, Morrice DJ, Clayton J, Mackay JH. Dynamic individual vital sign trajectory early warning score (DyniEWS) versus snapshot national early warning score (NEWS) for predicting postoperative deterioration. Resuscitation. 2020 Dec;157:176-184. doi: 10.1016/j.resuscitation.2020.10.037. Epub 2020 Nov 9. PMID: 33181231; PMCID: PMC7762721.
- Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, Huang DT, Kellum JA, Mi Q, Opal SM, Talisa V, van der Poll T, Visweswaran S, Vodovotz Y, Weiss JC, Yealy DM, Yende S, Angus DC. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019 May 28;321(20):2003-2017. doi: 10.1001/jama.2019.5791. PMID: 31104070; PMCID: PMC6537818.
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992 Jun;101(6):1644-55. doi: 10.1378/chest.101.6.1644. PMID: 1303622.
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003 Apr;29(4):530-8. doi: 10.1007/s00134-003-1662-x. Epub 2003 Mar 28. PMID: 12664219.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801-10. doi: 10.1001/jama.2016.0287. PMID: 26903338; PMCID: PMC4968574.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992 Jun;20(6):864-74. PMID: 1597042.
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005 Jan 1-7;365(9453):63-78. doi: 10.1016/S0140-6736(04)17667-8. PMID: 15639681.
- Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M; Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):775-87. doi: 10.1001/jama.2016.0289. PMID: 26903336; PMCID: PMC4910392.
- Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS, Escobar GJ, Angus DC. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):762-74. doi: 10.1001/jama.2016.0288. Erratum in: JAMA. 2016 May 24-31;315(20):2237. PMID: 26903335; PMCID: PMC5433435.
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003 Apr 17;348(16):1546-54. doi: 10.1056/NEJMoa022139. PMID: 12700374.
- Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome Criteria for the Diagnosis of Sepsis and Prediction of Mortality: A Systematic Review and Meta-Analysis. Chest. 2018 Mar;153(3):646-655. doi: 10.1016/j.chest.2017.12.015. Epub 2017 Dec 28. PMID: 29289687.
- Haydar S, Spanier M, Weems P, Wood S, Strout T. Comparison of QSOFA score and SIRS criteria as screening mechanisms for emergency department sepsis. Am J Emerg Med. 2017 Nov;35(11):1730-1733. doi: 10.1016/j.ajem.2017.07.001. Epub 2017 Jul 6. PMID: 28712645.
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368-77. doi: 10.1056/NEJMoa010307. PMID: 11794169.
- ProCESS Investigators; Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014 May 1;370(18):1683-93. doi: 10.1056/NEJMoa1401602. Epub 2014 Mar 18. PMID: 24635773; PMCID: PMC4101700.
- ARISE Investigators; ANZICS Clinical Trials Group; Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014 Oct 16;371(16):1496-506. doi: 10.1056/NEJMoa1404380. Epub 2014 Oct 1. PMID: 25272316.
- Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM; ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015 Apr 2;372(14):1301-11. doi: 10.1056/NEJMoa1500896. Epub 2015 Mar 17. PMID: 25776532.
- Angus DC, Barnato AE, Bell D, Bellomo R, Chong CR, Coats TJ, Davies A, Delaney A, Harrison DA, Holdgate A, Howe B, Huang DT, Iwashyna T, Kellum JA, Peake SL, Pike F, Reade MC, Rowan KM, Singer M, Webb SA, Weissfeld LA, Yealy DM, Young JD. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015 Sep;41(9):1549-60. doi: 10.1007/s00134-015-3822-1. Epub 2015 May 8. PMID: 25952825.
- PRISM Investigators; Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, Canter RR, Coats TJ, Delaney A, Gimbel E, Grieve RD, Harrison DA, Higgins AM, Howe B, Huang DT, Kellum JA, Mouncey PR, Music E, Peake SL, Pike F, Reade MC, Sadique MZ, Singer M, Yealy DM. Early, Goal-Directed Therapy for Septic Shock - A Patient-Level Meta-Analysis. N Engl J Med. 2017 Jun 8;376(23):2223-2234. doi: 10.1056/NEJMoa1701380. Epub 2017 Mar 21. PMID: 28320242.
- Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, Kluge S. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med. 2016 Feb;42(2):202-10. doi: 10.1007/s00134-015-4127-0. Epub 2015 Nov 10. PMID: 26556617.
- Theerawit P, Na Petvicharn C, Tangsujaritvijit V, Sutherasan Y. The Correlation Between Arterial Lactate and Venous Lactate in Patients With Sepsis and Septic Shock. J Intensive Care Med. 2018 Feb;33(2):116-120. doi: 10.1177/0885066616663169. Epub 2016 Aug 8. PMID: 27502951.
- Clec'h C, Fosse JP, Karoubi P, Vincent F, Chouahi I, Hamza L, Cupa M, Cohen Y. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit Care Med. 2006 Jan;34(1):102-7. doi: 10.1097/01.ccm.0000195012.54682.f3. PMID: 16374163.
- Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, Runyon MS, Self WH, Courtney DM, Nowak RM, Gaieski DF, Ebmeyer S, Johannes S, Wiemer JC, Schwabe A, Shapiro NI. Serial Procalcitonin Predicts Mortality in Severe Sepsis Patients: Results From the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit Care Med. 2017 May;45(5):781-789. doi: 10.1097/CCM.0000000000002321. PMID: 28257335; PMCID: PMC5389588.
- Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, Reinhart K, Selvakumar N, Levy MM. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015 Mar;43(3):567-73. doi: 10.1097/CCM.0000000000000742. PMID: 25479113.
- Tang Y, Choi J, Kim D, Tudtud-Hans L, Li J, Michel A, Baek H, Hurlow A, Wang C, Nguyen HB. Clinical predictors of adverse outcome in severe sepsis patients with lactate 2-4 mM admitted to the hospital. QJM. 2015 Apr;108(4):279-87. doi: 10.1093/qjmed/hcu186. Epub 2014 Sep 4. PMID: 25193540.
- Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010 Feb 24;303(8):739-46. doi: 10.1001/jama.2010.158. PMID: 20179283; PMCID: PMC2918907.
- Liu V, Morehouse JW, Soule J, Whippy A, Escobar GJ. Fluid volume, lactate values, and mortality in sepsis patients with intermediate lactate values. Ann Am Thorac Soc. 2013 Oct;10(5):466-73. doi: 10.1513/AnnalsATS.201304-099OC. PMID: 24004068; PMCID: PMC5475420.
- Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J; LACTATE study group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010 Sep 15;182(6):752-61. doi: 10.1164/rccm.200912-1918OC. Epub 2010 May 12. PMID: 20463176.
- Gu WJ, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med. 2015 Oct;41(10):1862-3. doi: 10.1007/s00134-015-3955-2. Epub 2015 Jul 8. PMID: 26154408.
- Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, Heard SO, Martin C, Napolitano LM, Susla GM, Totaro R, Vincent JL, Zanotti-Cavazzoni S. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004 Sep;32(9):1928-48. doi: 10.1097/01.ccm.0000139761.05492.d6. PMID: 15343024.
- Lindner HA, Balaban Ü, Sturm T, Weiß C, Thiel M, Schneider-Lindner V. An Algorithm for Systemic Inflammatory Response Syndrome Criteria-Based Prediction of Sepsis in a Polytrauma Cohort. Crit Care Med. 2016 Dec;44(12):2199-2207. doi: 10.1097/CCM.0000000000001955. PMID: 27441906.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
