Archives of Preventive Medicine
Re-emergence of malaria in Southern Italy: a remote possibility or a countdown?
1Collège Don Bosco, DBWSL, Brussels, Belgium
2CISSS Lanaudière, Direction de l’Enseignement et de l’Université de la Recherche, Joliette, Québec, Canada
3Louvain Drug Research Institute (LDRI), UCLouvain, Bruxelles, Belgium
Author and article information
Cite this as
Pezzulla A, SchioppaL, Poupaert JH. Re-emergence of malaria in Southern Italy: a remote possibility or a countdown?. Arch Prev Med. 2025; 10(1): 010-015. Available from: 10.17352/apm.000039
Copyright License
© 2025 Pezzulla A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Despite its eradication in 1970, southern Italy now confronts the limited but tangible prospect of malaria re-emergence, a concern shared by other warmer regions in Europe. The article discusses the rapidly changing climatic and societal conditions that are raising concerns about the potential resurgence of malaria in southern Europe, particularly in southern Italy. Malaria was endemic for millennia, and it was only in the second half of the 20th century that the disease was eradicated by disrupting the ecosystem of the Anopheles mosquito, which is the vector of the parasitic infection. This, combined with imported malaria cases and suitable environmental conditions, creates a significant risk for local transmission. As the conditions for re-emergence become increasingly favorable, and with the rise of resistance to antimalarial drugs in particular, are we prepared to counteract such a health crisis? The article discusses the primary risk factors of malaria re-emergence, with an emphasis on southern Italy, and suggests re-centering the debate around health professionals, rather than primarily on political actors.
Nowadays, malaria is mostly present in the Global South, notably in Africa. According to the WHO World Malaria Report 2024, there were an estimated 263 million malaria cases and 597,000 deaths worldwide in 2023, with more than 94% of cases and deaths occurring in the WHO African Region [1]. However, malaria also has a deep and historically significant presence across Europe, particularly in the southern regions, where it once constituted a major public health burden. Although successfully eradicated, leading to a diminished perception of its threat, the re-emergence of mosquito-borne diseases like malaria and dengue in Europe necessitates renewed vigilance [2].
According to the ECDC, there were 6,131 confirmed cases in 2022 across Europe, of which 99.8% were imported. Only 13 cases were locally acquired: seven in France, three in Germany, two in Spain, and one in Ireland [3]. Factors such as climate change and increased international travel strongly support the critical scientific discussion regarding the risk of malaria’s re-establishment, which our paper further explores. It is generally accepted that European countries are currently safe from a return of malaria, including in the southern regions of the Old Continent. This would be due to the great Saharan barrier, which supposedly efficiently blocks mosquitoes’ access from entering from West Africa, where malaria is endemic. However, the climate changes that are shaking up the climatology of the temperate zones of the Northern Hemisphere are rapidly creating a critical new situation that should be carefully examined [4].
One of the earliest historical testimonies of the deleterious presence of malaria was written on the tomb of the great Roman poet Virgil: “Mantua me genuit; Calabri rapuere; tenet nunc Parthenope. “Mantua gave me life, the Calabrians took it away, Naples holds me now.” The poet presumably died from malaria on his way back from Greece. Some unverifiable hypotheses claim that malaria has killed nearly half of all human beings on Earth. This is clearly excessive. However, the disease nowadays primarily kills children: an estimated figure of 600,000 annual malaria deaths, two-thirds of which are children under the age of 5 [5]. It is one of the worst scourges in human history, persisting for millennia. Malaria is caused by a parasite, Plasmodium, which is transmitted by a specific species of mosquito: the Anopheles mosquito. Symptoms typically include fever, headache, and chills, and if left untreated, particularly the Plasmodium falciparum type, it can progress to severe illness and death.
Beyond the genetic pressure on humanity, malaria and yellow fever have played a crucial role in our history. They have determined the outcome of certain wars and conflicts, and conditioned some of the invasions that have shaped our geography, up to the present day. Malaria, to some extent, protected sub-Saharan Africa from colonial invasions until the 19th century, while the colonization of Latin America by Europe took place as early as the 16th century. It was most likely the Europeans who brought malaria to Latin America, which was previously untouched by these fevers. As soon as the colonists advanced beyond the Maghreb, they faced a significant mortality rate. It was from the moment quinine was recognized as a medicine against brook fevers that the colonization of Africa began.
From war to war, new molecules are discovered, most notably artemisinin, derived from the Artemisia plant. By exhuming ancient manuscripts of traditional medicine, Chinese researcher To Youyou discovered the secret to its effectiveness and was awarded the Nobel Prize in Medicine in 2015 [6]. Nevertheless, to identify the direct cause of the disease, we must go back to the end of the 19th century, with the discovery of Plasmodium as a malaria agent by French Alphonse Laveran in 1880. The link to vectors such as Anopheles mosquitoes was identified by Englishman Ronald Ross in 1895. Both would receive the Nobel Prize [7].
Thanks to the identification of the role of mosquitoes, at the beginning of the 20th century, American surgeon William C. Gorgas led an unprecedented fight against potential mosquito breeding grounds. This fight would lead to improved living conditions on the Panama Canal construction site. By 1969, the year man first set foot on the moon, the WHO recognized that malaria was becoming resistant to DDT [8]. This growing resistance prompted renewed global efforts, and beginning in the 1990s, under pressure from philanthropists such as Bill Gates, the WHO launched a new malaria control initiative called “Roll Back Malaria” [9].
However, the history of malaria in Europe extends much earlier: by the late 19th century, in about a third of Italy’s territory, malaria had already become widespread, affecting approximately 10% of the population and causing 15,000–20,000 deaths annually out of 2 million cases. Mortality rates were particularly high in the central (e.g., Maremma in Tuscany, Agro Romano), southern regions, and islands, including the Tyrrhenian and Ionian coasts, Sardinia, Calabria, and Sicily. Plasmodium falciparum (the deadliest species of malaria parasite) was the most prevalent in these areas, while in the North, milder forms caused by P. vivax and P. malariae were more common [10,11]. Although malaria was declared eradicated in Italy in 1970, the ecological and social determinants that once sustained endemic transmission—such as the presence of competent Anopheles vectors and human mobility from endemic regions—are gradually re-emerging [12]. It is nowadays the most important imported tropical disease. That is why some measures are still taken to monitor its epidemiology; notably the fact that the notification of each case—imported or autochthonous—is still mandatory. However, the Italian Ministry of Health acknowledges that this measure presents some weaknesses. In fact, malaria cases are likely underestimated in certain regions, primarily because every diagnosis requires microscopic confirmation, and many of these confirmations are never officially reported. Furthermore, notifications are sometimes delayed, even though diagnoses of imported cases must be reported within 5 days and autochthonous cases within 24 hours. In some instances, these delays can extend to several months, reducing the effectiveness of surveillance and control measures. This underreporting is particularly concerning because malaria is mainly transmitted by mosquito vectors, but it can also be spread through other human-to-human routes, such as blood transfusions. Accurate and timely reporting is therefore crucial to prevent both vector-borne and non-vector-borne transmission and to ensure the safety of transfusion products by specialized doctors in Italy.
Furthermore, the patients who are suspected of being infected with malaria undergo malaria screening, which consists of a specific survey and an immunological test [13]. Nevertheless, these measures do not always prevent nosocomial infections of malaria. We can notably refer to the two nosocomial infections of 2017 [14]. However, vectorial transmission is not to be completely excluded from the possibilities. For instance, in 1997, in the province of Grosseto, a person was contaminated with P. vivax malaria from an indigenous An. Labranchiae [13]. For further monitoring of imported cases, medical strategies should also include targeted screening among immigrants and travelers from endemic regions using peripheral blood smears or rapid diagnostic tests [15].
Importantly, malaria is not only a modern concern in Italy. Ancient DNA studies have revealed the presence of P. falciparum in Southern Italy as early as the 1st–2nd century CE [16], demonstrating that the disease has had a long-standing impact on the region across various ecological and cultural contexts. This historical perspective underscores the persistent challenges in controlling malaria, from ancient times to present-day nosocomial and vector-borne cases.
Discussion
As we have just seen, the fate of humanity since its origins and the survival of certain species of Anopheles are based on a form of saprophytism of mosquitoes and Homo sapiens. The recent eradication of malaria in southern Europe, in particular, was the result of strong ecological pressure against mosquitoes in these regions. Can this result be considered definitive, or could it be called into question by environmental changes? This eradication was the result, among other things, of the intensive use of pesticides, including the notorious DDT. Would such a practice still be acceptable today from an ecological, or even simply moral, point of view? That is one of the questions. If malaria was endemic in these regions, it is because there was a favorable environment there that still exists and that we have simply destabilized for a while. One may wonder whether the current climate change caused by the industrial era is likely to influence the re-emergence of malaria in certain regions, which are at the interface between endemic infested areas and others further north, which are immune due to their colder and therefore unfavorable climate. Predictive models combining climate, entomological, and demographic data suggest that suitable habitats for Anopheles mosquitoes are likely to expand northward in Europe, increasing receptivity to local transmission under future climate scenarios [17]. In fact, between 2010 and 2020, clusters of locally transmitted malaria were documented in Greece and Spain, underlining that favorable ecological and social conditions can still enable transmission in Europe [18]. A similar concern arises when examining the climate in the southern Calabria region, where there is a noticeable rarefaction of the rainfall regime in spring and autumn, which leads to a slow desertification, particularly along the coast of the Ionian Sea, accompanied, however, by more intense rainy episodes than before [19]. Meteorological measurements indicate a rather weak trend towards warming with many local variations. Where this phenomenon is more marked is in the mountains (Aspromonte). Given that the development of Anopheles mosquitoes is conditioned by high temperatures (at least 16°C) and the presence of puddles, one might say that the current trend is not, a priori, towards a resurgence of malaria. The temperatures currently observed throughout the year point towards an extension of favorable periods, even in the mountains. Furthermore, the more frequent stormy episodes [20], even in summer, create a climatic continuum favorable to mosquito species already endemic in these regions and to the arrival of other, more dangerous species, as they carry Plasmodium. In this regard, we recall the appearance of tiger mosquitoes in Italy in recent decades.
Apart from these climatic aspects, what can be added? Since the declared eradication, over the last fifty years, sporadic cases of malaria in Italy in general (500-700 cases per year) are of an imported nature, for example, by travelers returning from endemic areas, such as West Africa [21]. This national trend reflects a broader European pattern, as imported malaria cases in the EU/EEA show a distinct seasonal peak between July and September, largely linked to summer travel to endemic areas [3]. While this travel-related malaria is relatively well characterized and generally contained, a further element that deserves attention is the growing demographic pressure from Africa towards Europe. This movement, often driven by economic migration, can also contribute to the introduction of infected individuals, although the magnitude of this phenomenon remains insufficiently documented. This constitutes an element of potential destabilization of the metastable state of malaria in southern Europe. Despite that, it’s important to note that there were recent reports of autochthonous malaria clusters in Italy, which also suggest a re-evaluation and potential update of the existing malaria surveillance system [14,22].
In addition to all of the above, two more elements should also be brought to the table; on the one hand, the rise in resistance of Plasmodium to existing antimalarials, including artemisinin derivatives (artesunate, for example), and the level of preparedness of health bodies [23]. The increasing resistance to artemisinin represents a true threat, because the current first- and second-line treatment against uncomplicated malaria caused by P. falciparum or chloroquine-resistant P. vivax are Artemisinin-based Combination Therapies (ACTs), that is, an association of a derivative of artemisinin—such as artesunate or artemether—with another antimalarial drug—such as amodiaquine or lumefantrine. It is interesting to know that the resistance to artemisinins is only “partial”, which means that it only occurs during a specific stage of the parasitic cycle: the ring stage. The main consequence of this resistance is a delay in the clearance of the parasites from the blood. No “full” resistance to artemisinins has ever been reported so far. Therefore, if the Plasmodium is resistant only to artemisinins, ACTs should still be effective if the associated drug is still efficacious. However, some strains in the Great Mekong Subregion seem to have developed a resistance to both the artemisinin derivative and the associated drug of some of the ACTs that are used in their regions. This obviously fails these ACTs. When this happens, it is certainly possible to change the combination of the drugs—in other words, using another ACT. Furthermore, it is interesting to note that, often, the resistance to antimalarial treatments can be developed by several different strains of Plasmodium independently [24]. This leads us to wonder how long artemisinin and its derivatives will be efficient before they become completely ineffective, as other drugs that were used against malaria before ACTs. For example, we can mention Mefloquine. This drug used to be the first-line treatment against malaria, but it became completely ineffective within 6 years after its introduction [25]. If a similar situation were to happen with artemisinin, it would be a serious disaster. The erosion of the effectiveness of medicinal agents is not specific to this class of drugs, and it is hoped that pharmaceutical companies will tackle the problem head-on soon. Ongoing research on drug resistance, both in vivo and in vitro, is essential to anticipate treatment failures and guide therapeutic strategies [26].
The apparent victory against malaria in southern Europe has created a form of negligence among the public and, consequently, among politicians. There is talk of inactive surveillance networks, and what about the level of preparedness of the rank-and-file physician? General practitioners are primarily trained to prescribe preventive medication for travelers. However, given the diffuse symptoms, especially at the beginning of the infection, this makes diagnosis more difficult, especially in areas of southern Europe with little or no travel.
The sporadic nature of these difficult-to-diagnose cases in southern Europe finds an interesting analogue in Florida. In this state, malaria has not been endemic since the 1940s. Nevertheless, just as for Italy, very few homegrown cases still occur sporadically [14]. However, there is an interesting difference between Italy and Florida: while vectors are very rare in Italy, it is not in Florida. In fact, there are still 8 species of Anopheles that are active and abundant in this state, notably Anopheles Quadrimaculatus [27]. Thus, contrary to Europe, locally-acquired malaria in Florida may not only be the result of human-to-human transmission, but also of a vectorial transmission [28]. However, the presence of these vectors is not likely to result in a major outbreak for now because of the combination of two factors: firstly, there are still very few malaria cases in the United States (imported or homegrown), which means that it is very improbable for mosquitoes to bite an infected person. Secondly, the mosquitoes that are present in Florida are not highly anthropophilic, which means that they prefer to bite other animals rather than humans. We could thus affirm that these vectors do not represent a threat for now. In fact, this huge population of mosquitoes is not controlled anymore because of the low risk of the re-emergence of malaria. Moreover, another factor that prevents malaria outbreaks is the cold of winter, which hinders proliferation during this period. However, climate change is leading to warmer winters. This means that the situation of vectors in Florida demands ongoing surveillance, as the shifting climate could fundamentally alter the current risk assessment for malaria re-emergence, mirroring the climate-driven concerns also emerging for southern Italy [29]. Environmental interventions such as draining stagnant waters, improving urban drainage, and larviciding remain crucial for vector control in receptive regions [30].
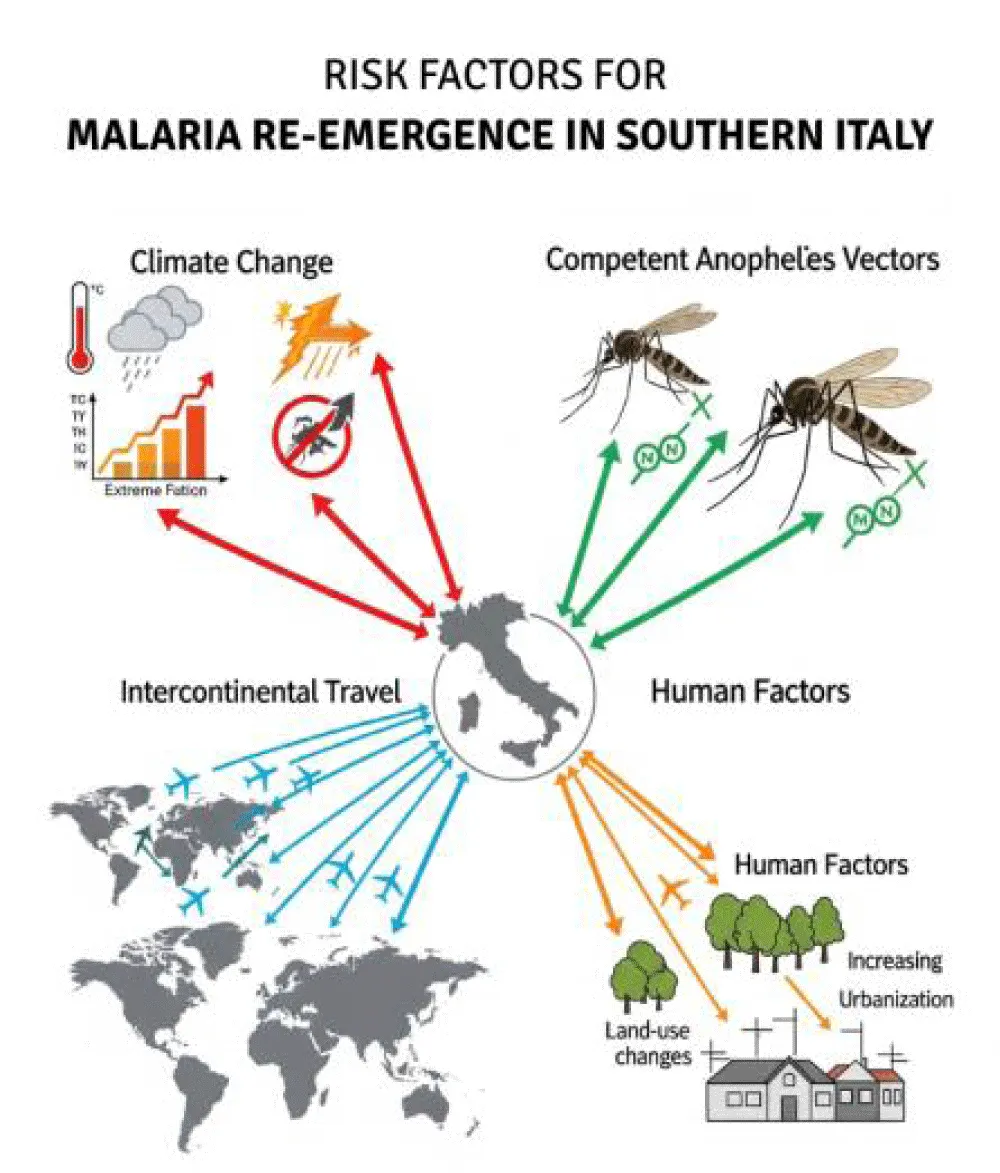
The purpose of this note is not to be yet another message in a bottle thrown into an ocean of information, more or less well filtered by artificial intelligence. This brief communication is an essay intended as a note of hope. Let us explain. So far, the catastrophe has not (yet) occurred. And we can still act, but what can we do? We cannot decisively influence the climate in a short time, despite what some people may say. We cannot effectively prevent the rise of Plasmodium’s resistance to existing antimalarial drugs. And it would be unrealistic to limit more frequent intercontinental travels, or to influence migratory flows across the Mediterranean. Furthermore, the human population itself and its interactions with the environment and mosquitoes determine exposure and susceptibility to infection if the other factors align (Figure 1). Indeed, climatic changes, the presence of competent Anopheles vectors, intercontinental travel, and anthropogenic factors such as land-use changes and urbanization all converge to create a multifaceted risk scenario for malaria re-emergence in Southern Italy. So what does this mean in practice? We can already consolidate what exists, yet strengthening malaria preparedness does not always seem to be on the agenda. One concrete measure could be the reactivation of surveillance networks through general practitioners, for instance, by extending training sessions that already exist for other medical subjects [31]. We must continue to emphasize the need for good prophylaxis before embarking on a trip to an infected area. Ultimately, a truly comprehensive strategy against malaria reintroduction must integrate environmental control, enhanced clinical vigilance, robust public health preparedness, and ongoing research on drug and insecticide resistance [32]. Within this framework, at the center of this debate, their role in early detection, treatment, and community education is crucial to preventing malaria re-establishment in Europe [33].
This may seem obvious, but how many times have we heard someone say they traveled without taking their medication and didn’t catch anything? It’s like saying I drive my car without wearing a seatbelt. It is certainly important to be vaccinated against yellow fever before traveling to intertropical countries because it is required by law. However, malaria prophylaxis is left to your discretion. Non-imported malaria means fewer chances of non-spreading, even in metastable areas (for example, southern Italy). Let us recall in this regard that vector transmission of malaria occurs through certain Anopheles species based on a vicious circle: an infected mosquito bites a human who becomes infected, who in turn is bitten again by another mosquito who in turn becomes infected. If this scenario is not fully followed, the infection cannot be maintained and further progress [34].
Conclusions
Malaria has a deep and historically significant presence in Europe and Italy, particularly in the southern regions, where it long constituted a major public health burden. However, despite its successful eradication and a diminishing perception of its threat, the risk of its re-establishment remains a crucial topic of scientific discussion, a concern we strongly support given factors such as climate change and increased international travel [2]. If re-infestation were to occur gradually, health authorities, with the assistance of public health bodies, would likely have the necessary time to implement adaptive strategies [35]. However, if a seemingly fortuitous event were to occur that destabilizes the currently unstable ecosystem (invasion of a new species of Anopheles, for example), one can reasonably fear the development of a state of crisis. Who could have predicted what happened with the COVID-19 pandemic and the extension it took? Malaria must be compared with dengue fever and chikungunya, which are also mosquito-borne infections [36]. Nowadays, these last two pathologies seem to receive more interest from the media while the three threats coexist. Is this justified? In conclusion, the risk of malaria re-emergence in Italy—and more generally across Southern Europe—is a subject of ongoing scientific debate. Within this discussion, while the likelihood of a widespread epidemic is generally dismissed, several factors contributing to a low but persistent risk of re-emergence have been identified in this article, demonstrating that the situation warrants continuous epidemiologic surveillance. Ultimately, time will be the great judge of what will happen in the future of the futures.
Author contributions: Conceptualization, A.P. and J.H.P.; resources, A.P., L.S., and J.H.P.; writing—original draft preparation, A.P., L.S., and J.H.P.; writing—review and editing, L.S., A.P., and J.H.P.; supervision, J.H.P.; project administration, J.H.P. All authors have read and agreed to the published version of the manuscript.
Conflict of interest: The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
- World Health Organization. World Malaria Report 2024. Geneva: World Health Organization; 2024. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024
- Bueno R, Jiménez R. Re-Emergence of Malaria and Dengue in Europe [Internet]. In: Current Topics in Tropical Medicine. InTech; 2012. Available from: http://dx.doi.org/10.5772/26178
- European Centre for Disease Prevention and Control. Malaria – Annual Epidemiological Report for 2022. Stockholm: ECDC; 2023. Available from: https://www.ecdc.europa.eu/en/publications-data/malaria-annual-epidemiological-report-2022
- Grant L, Vanderkelen I, Gudmundsson L, Fischer E, Seneviratne SI, Thiery W. Global emergence of unprecedented lifetime exposure to climate extremes. Nature. 2025;641(8062):374-9. Available from: https://doi.org/10.1038/s41586-025-08907-1
- Le paludisme, tueur numéro un depuis la préhistoire : épisode 1/4 du podcast Mécaniques des épidémies, saison 5 : le paludisme | France Culture [Internet]. [cited 2025 ]. Available from: https://www.radiofrance.fr/franceculture/podcasts/mecaniques-des-epidemies/le-paludisme-tueur-numero-un-depuis-la-prehistoire-9205812
- Su XZ, Miller LH. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci China Life Sci. 2015 Nov;58(11):1175–9. Available from: https://doi.org/10.1007/s11427-015-4948-7
- Cox FE. History of the discovery of the malaria parasites and their vectors. Parasites Vectors. 2010 Feb;3(1):5. Available from: https://doi.org/10.1186/1756-3305-3-5
- Nájera JA, González-Silva M, Alonso PL. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969). PLoS Med. 2011;8(1):e1000412. Available from: https://doi.org/10.1371/journal.pmed.1000412
- Nabarro DN, Tayler EM. The "Roll Back Malaria" Campaign. Science. 1998;280(5372):2067–8. Available from: https://doi.org/10.1126/science.280.5372.2067
- Majori G. Short history of malaria and its eradication in Italy with short notes on the fight against the infection in the Mediterranean basin. Mediterr J Hematol Infect Dis. 2012;4(1):e2012016. Available from: https://doi.org/10.4084/mjhid.2012.016
- Snowden FM. The Conquest of Malaria: Italy, 1900-1962. Conqu Malar [Internet]. 2006 Jan 14 [cited 2025 Jun 28]; Available from: https://academic.oup.com/yale-scholarship-online/book/23095, https://www.researchgate.net/publication/287721860_The_Conquest_of_Malaria_Italy_1900-1962
- Romi R. History and updating on the spread of malaria in Italy. Parassitologia. 1999;41(1–3):31–8.
- Ministero della Salute. Prevenzione e controllo della malaria in Italia. Esenzioni. 2016;0036391–27:23. Available from: https://www.seremi.it/sites/default/files/Prevenzione%20e%20controllo%20della%20malaria%20in%20Italia%20dicembre%202016.pdf
- Boccolini D, Menegon M, Di Luca M, Toma L, Severini F, Marucci G, et al. Non-imported malaria in Italy: Paradigmatic approaches and public health implications following an unusual cluster of cases in 2017. BMC Public Health [Internet]. 2020 Jun 5;20(1):1–12. Available from: https://doi.org/10.1186/s12889-020-08748-9
- European Centre for Disease Prevention and Control. Guidelines for the prevention of travel-associated malaria. Stockholm: ECDC; 2019. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/AER-malaria-2019.pdf
- Marciniak S, Prowse TL, Herring DA, Klunk J, Kuch M, Duggan AT, et al. Plasmodium falciparum malaria in the 1st–2nd century CE in southern Italy. Curr Biol. 2016;26(23):R1220–2. Available from: https://www.researchgate.net/publication/311449318_Plasmodium_falciparum_malaria_in_1st-2nd_century_CE_southern_Italy
- Proestos Y, Christophides GK, Ergüler K, Tanarhte M, Waldock J, Lelieveld J. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Philos Trans R Soc Lond B Biol Sci. 2015;370(1665):20130554. Available from: https://doi.org/10.1098/rstb.2013.0554
- Danis K, Baka A, Lenglet A, Van Bortel W, Terzaki I, Tseroni M, et al. Malaria in Greece: historical and current reflections on a re-emerging vector-borne disease. Malar J. 2011;10:239. Available from: https://pubmed.ncbi.nlm.nih.gov/22027375/
- Istituto di Ricerca per la Protezione Idrogeologica. Siccità, desertificazione e cambiamenti climatici in Calabria [Internet]. [cited 2025 Jun 25]. Available from: https://www.irpi.cnr.it/focus/siccita-desertificazione-e-cambiamenti-climatici-in-calabria/
- Barbaro G, Bombino G, Foti G, Barillà GC, Puntorieri P, Mancuso P. Possible Increases in Floodable Areas Due to Climate Change: The Case Study of Calabria (Italy). Water (Switzerland). 2022;14(14):1–19. Available from: https://www.mdpi.com/2073-4441/14/14/2240
- Biyela S. Le changement climatique et les migrations empêchent les efforts d’éliminer le paludisme. Nat Africa [Internet]. 2024 [cited 2025 ]; Available from: https://doi.org/10.1038/d44148-024-00133-7
- Benelli G, Pombi M, Otranto D. Malaria in Italy – Migrants Are Not the Cause. Trends Parasitol [Internet]. 2018 May 1 [cited 2025];34(5):351–4. Available from: https://doi.org/10.1016/j.pt.2018.01.002
- Résistances aux antipaludiques - CNR du paludisme [Internet]. [cited 2025 Jun 25]. Available from: https://cnr-paludisme.fr/activites-dexpertise/resistances-aux-antipaludiques/
- Malaria: Artemisinin partial resistance [Internet]. [cited 2025]. Available from: https://www.who.int/news-room/questions-and-answers/item/artemisinin-resistance
- Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet [Internet]. 2004 Jul 31 [cited 2025];364(9432):438–47. Available from: https://pubmed.ncbi.nlm.nih.gov/15288742/
- Adam M, Nahzat S, Kakar Q, Assada M, Witkowski B, Tag Eldin Elshafie A, et al. World Health Organization. Antimalarial drug resistance. Geneva: WHO; 2023. Available from: https://doi.org/10.1111/tmi.13929
- Florida Department of Health. Florida Department of Health Mosquito-Borne Disease Guidebook: Chapter 8 Malaria Background. 2019;1–8. Available from: https://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/guidebook.html
- Malaria | Florida Department of Health [Internet]. [cited 2025]. Available from: https://www.floridahealth.gov/diseases-and-conditions/malaria/index.html#content_container
- Malaria’s Comeback in the U.S. | Johns Hopkins | Bloomberg School of Public Health [Internet]. [cited 2025]. Available from: https://publichealth.jhu.edu/2023/malarias-comeback-in-the-us. Available from: https://www.cdc.gov/malaria/php/surveillance-report/index.html
- WHO. Handbook for Integrated Vector Management. Geneva: World Health Organization; 2012. Available from: https://www.who.int/publications/i/item/9789241502801
- NOUL Blog | Recent Native Case of Malaria in Italy in Decades since the 1960s – What It Means for Public Health [Internet]. [cited 2025]. Available from: https://noul.com/en/board_news_blog/malaria-in-italy-first-native-case/
- WHO. Global Technical Strategy for Malaria 2016–2030, 2021 update. Geneva: World Health Organization; 2021. Available from: https://www.who.int/publications/i/item/9789240031357
- European Centre for Disease Prevention and Control (ECDC). Vector-borne diseases in Europe: 2020 epidemiological update. Stockholm: ECDC; 2021. Available from: https://www.ecdc.europa.eu/en/publications-data/organisation-vector-surveillance-and-control-europe
- Romi R, Sabatinelli G, Majori G. Could Malaria Reappear in Italy? Emerg Infect Dis [Internet]. 2001 [cited 2025];7(6):915–9. Available from: https://doi.org/10.3201/eid0706.010601
- Tornerà la malaria in Italia? L’esperto ‘no allarme ma guardia alta’ - Il Giornale d’Italia [Internet]. [cited 2025 ]. Available from: https://www.ilgiornaleditalia.it/news/salute/604606/tornera-la-malaria-in-italia-l-esperto-no-allarme-ma-guardia-alta.html
- Desmecht D, Hayette MP, Darcis G. Selon une étude, la dengue et le chikungunya pourraient s’installer durablement en Europe : faut-il s’inquiéter ? [Internet]. [cited 2025]. Available from: https://www.tameteo.com/actualites/actualite/selon-une-etude-la-dengue-et-le-chikungunya-pourraient-s-installer-durablement-en-europe-faut-il-s-inquieter-moustique-tigre-endemiques-maladie-sante-rechauffement-climatique.html
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
