Archives of Preventive Medicine
Influence of Herbal Co-administration on Metformin Dissolution: A UV Spectroscopic Study of Drug-Food Interaction with Carica Papaya leaf
15th Pharm d student, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, College of Pharmacy, Coimbatore – 641044, India
2Professor, Department of Pharmaceutics, Sri Ramakrishna Institute of Paramedical Sciences, College of Pharmacy, Coimbatore – 641044, India
Author and article information
Cite this as
Mirdula Mary E, Bagyalakshmi J. Influence of Herbal Co-administration on Metformin Dissolution: A UV Spectroscopic Study of Drug-Food Interaction with Carica Papaya leaf. Arch Prev Med. 2025; 10(1):006-009. Available from: 10.17352/apm.000038
Copyright License
© 2025 Mirdula Mary E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction
Type 2 Diabetes Mellitus (T2DM) is a growing global health concern, with India bearing a significant share of the burden. Among oral antidiabetic agents, metformin remains the first-line therapy due to its efficacy in lowering blood glucose, primarily by inhibiting hepatic gluconeogenesis and improving insulin sensitivity (Młynarska, n.d.) [1]. It is commonly prescribed alongside sulfonylureas, especially in resource-constrained settings. However, recent years have witnessed an increasing use of newer agents like SGLT2 inhibitors, particularly in patients with coexisting cardiovascular or renal conditions, due to their additional cardiometabolic benefits (Dutka, n.d.) [2]. Parallel to advancements in synthetic pharmacotherapy, there is renewed interest in traditional medicinal plants (Bailey, n.d.) [3]. Carica papaya, widely used in folk medicine, has shown promising antidiabetic effects in preclinical studies (Kazeem, n.d.) [4]. Extracts from its leaves have demonstrated potential in reducing blood glucose levels, enhancing insulin secretion, and supporting pancreatic β-cell regeneration (Green synthesis and characterization of silver nanoparticles using Cassia fistula and assessment of its in vitro anti diabetic activity. J Bagyalakshmi, n.d.) [5]. This article explores the evolving landscape of T2DM management, highlighting the integration of established drugs like metformin with emerging therapies, including plant-based alternatives such as Carica papaya. (Kumarasinghe, n.d.) [6]. It is rich in vitamins (A, C, E), minerals, enzymes, and phytochemicals such as flavonoids, phenolic acids, carotenoids, and alkaloids. These contribute to its antioxidant, anti-aging, anti-inflammatory, and anticancer properties (Kumarasinghe, n.d.) [6]. Due to the bioactive components (carpaines, BITC, benzyl glucosinolates, latex, papain, zeaxanthin, choline, etc.) in its seeds, leaves, and fruits, it is revered for its excellent antioxidant, digestive, and nutraceutical benefits. Papayas are high in vitamins A, B, C, E, and K, folate, pantothenic acid, zeaxanthin, lycopene, lutein, magnesium, copper, calcium, and potassium (Agada, n.d.) [7]. Being rich in fiber, antioxidants, and vitamin C, it lowers the cholesterol in the arteries; prevents arthritis; reduces aging, cancer, macular degradation, risk of cardiovascular diseases, and stress; increases platelet count; controls dengue fever; facilitates digestion, and lowers body weight. Papaya leaf extract, with many in vitro and case studies in combination therapies with modern medicine, especially for cancers and many other viral diseases, is an efficient cure. (Koul, n.d.) [8].
Objectives
The primary aim of this study is to investigate the potential interaction between Carica papaya extract and Metformin Hydrochloride (HCl), with a specific focus on dissolution kinetics, UV absorbance behavior, and non-compartmental pharmacokinetic modeling. (Janardhanan, n.d.) [9] This exploration is motivated by the increasing co-administration of herbal extracts with conventional oral antidiabetic agents and the possibility of altered pharmacokinetic behavior, which can impact both drug efficacy and safety (Abdelgawad, n.d.) [10].
- To assess whether Carica papaya extract alters the dissolution kinetics of Metformin Hydrochloride.
- To determine whether the presence of Carica papaya extract affects the UV absorbance of metformin during dissolution.
- To model the dissolution data using a non-compartmental pharmacokinetic approach and compare kinetic parameters with and without Carica papaya.
Methodology
Fresh Carica papaya leaves were washed, shade-dried, and coarsely powdered. An aqueous extract was prepared via steam distillation using distilled water. The distillate was filtered, concentrated using a rotary evaporator, and stored at 4 °C in amber bottles until use. (Bagyalakshmi, n.d.) [11].
Dissolution of metformin hydrochloride tablets was performed using the USP Type II (paddle) apparatus in 900 mL of two media:
- Simulated gastric fluid (pH 1.2).
- Phosphate buffer (pH 7.4).
Conditions were maintained at 37 ± 0.5 °C with a paddle speed of 50 rpm.
Two groups were tested:
- Group A: Metformin alone.
5 mL samples were withdrawn at 0, 5, 10, 15, 30, 45, 60, and 75 minutes, filtered, and replaced with fresh medium. Metformin concentrations were measured using UV-Visible spectrophotometry at 236 nm. Calibration curves were prepared in both media. Each measurement was done in triplicate, and results were expressed as mean ± SD. Dissolution profiles (% drug release vs. time) were compared between groups. Non-compartmental pharmacokinetic analysis was applied to determine: Cₘₐₓ, Tₘₐₓ, AUC₀, ₇₅min, and Mean Dissolution Time (MDT) (Bagyalakshmi, n.d.) [12].
- Group B: Metformin with Carica papaya extract (accordingly change - mixture).
5 mL samples were withdrawn at 0, 5, 10, 15, 30, 45, 60, and 75 minutes, filtered, and replaced with fresh medium. Metformin concentrations were measured using UV-Visible spectrophotometry at 236 nm (Metry, n.d.) [12]. Calibration curves were prepared in both media. Each measurement was done in triplicate, and results were expressed as mean ± SD. Dissolution profiles (% drug release vs. time) were compared between groups. Non-compartmental pharmacokinetic analysis was applied to determine: Cₘₐₓ, Tₘₐₓ, AUC₀, ₇₅min, and Mean Dissolution Time (MDT) [11].
Results
- Group A: Metformin alone
The dissolution profile of metformin hydrochloride was studied using the USP Type II dissolution apparatus (paddle method), in compliance with pharmacopeial standards for oral solid dosage forms. The paddle speed was maintained at 50 rpm throughout the experiment to ensure uniform mixing and to mimic mild gastrointestinal motility. The concentration of metformin hydrochloride in each sample was measured using a UV-Visible spectrophotometer. The absorbance was recorded at 236 nm, the wavelength corresponding to the λₘₐₓ of metformin.
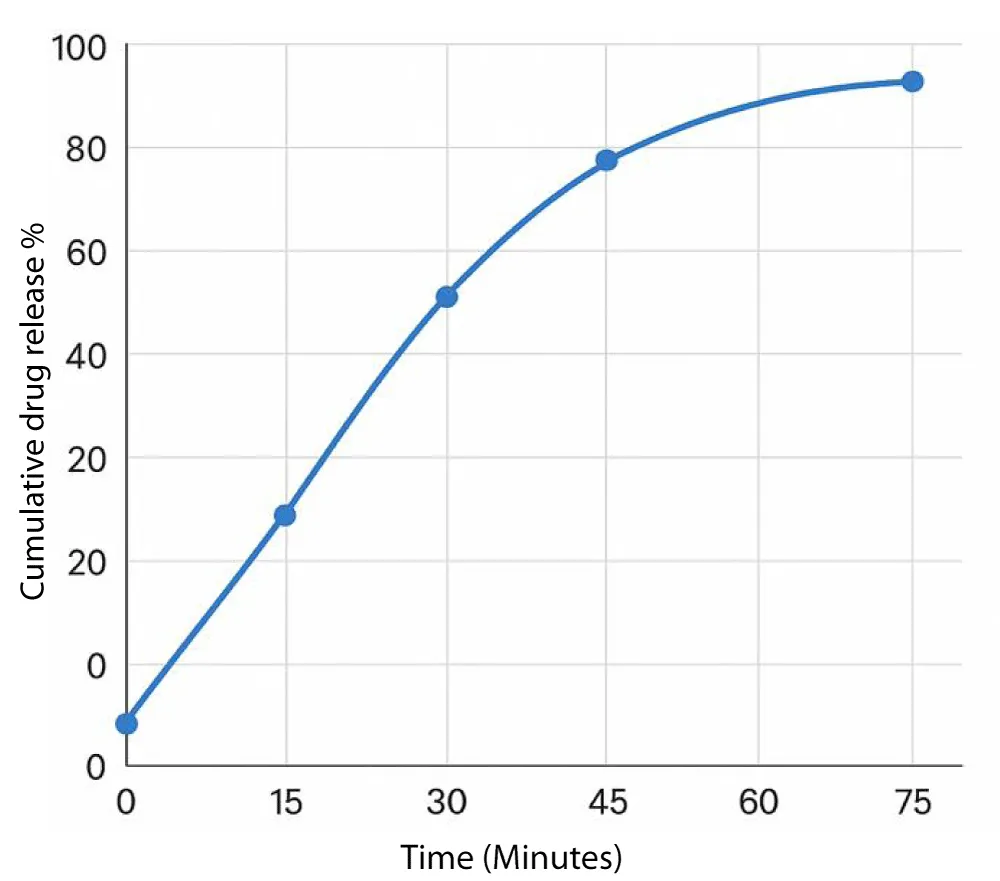
The dissolution profile of metformin hydrochloride is displayed in the following graph (Figure 1).
The dissolution profile of metformin in buffer shows a steady increase in cumulative drug release with time. At 15 minutes, approximately 35% of the drug is released, which rises to nearly 65% by 30 minutes. A further increase to about 85% is observed at 45 minutes. The release then gradually approaches a plateau, reaching close to 95% by 75 minutes, indicating almost complete dissolution [13].
- Group B: Metformin with Carica papaya extract
The dissolution profile of metformin hydrochloride was studied using the USP Type II dissolution apparatus (paddle method), in compliance with pharmacopeial standards for oral solid dosage forms. The paddle speed was maintained at 50 rpm throughout the experiment to ensure uniform mixing and to mimic mild gastrointestinal motility. The concentration of metformin hydrochloride in each sample was measured using a UV-Visible spectrophotometer. The absorbance was recorded at 236 nm, the wavelength corresponding to the λₘₐₓ of metformin. (Gupta, n.d.) [13].
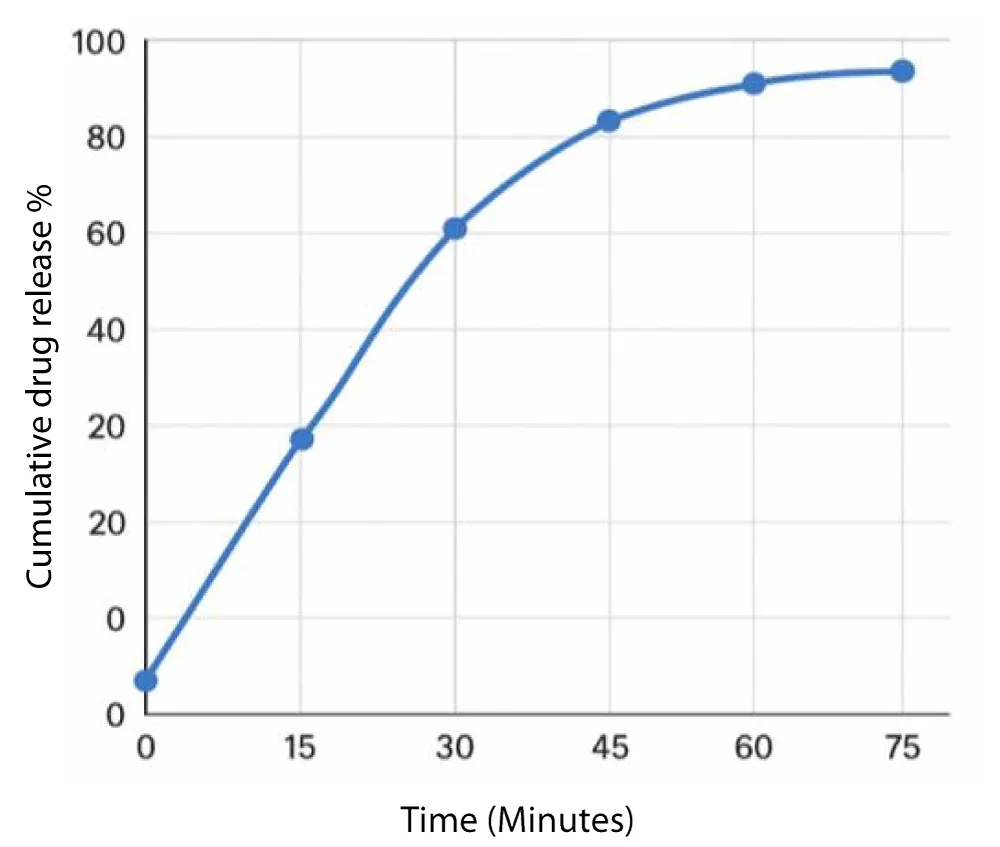
The dissolution profile of metformin hydrochloride is displayed in the following graph (Figure 2).
The dissolution profile of metformin in the presence of Carica papaya leaf extract exhibited a slower initial release compared to metformin alone. At 15 minutes, only about 28% of the drug was released, in contrast to nearly 35% in the control. By 30 minutes, the release increased to approximately 52%, which remained lower than the 65% observed with metformin alone. The release continued to rise gradually, reaching around 92% by 75 minutes. Overall, the presence of Carica papaya leaf extract resulted in a delayed and reduced dissolution rate. (Gupta, n.d.) [13] (Table 1).
The in vitro dissolution study revealed a noticeable difference in drug release profiles between metformin (Zhou, n.d.) [14] administered alone and in combination with Carica papaya leaf extract. Specifically, the area under the curve from 0 to 75 minutes (AUC₀–75) for metformin alone was 4582.5 %·min, while the AUC for the metformin with papaya extract group was 4155.0 %·min, indicating a reduction of approximately 9.3% in total drug exposure over the observed time period. Similarly, the mean concentration (C̄) of metformin during dissolution decreased from 61.10% in the control group to 55.40% in the test group [13]. This decline suggests that the co-administration of Carica papaya extract may have interfered with the dissolution or release rate of metformin, potentially due to interactions between the plant constituents and the drug, such as changes in solubility, pH microenvironment, or binding interactions (Fakeye, n.d.) [15]. These findings imply a moderate inhibitory effect of the papaya extract on metformin’s dissolution kinetics, which could have implications for its bioavailability if co-administered in vivo. (Bagyalakshmi, n.d.) [11].
Discussion
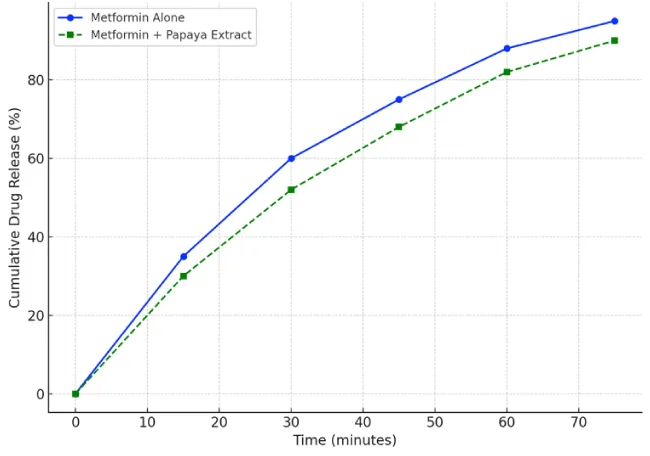
Initial Phase 0–15 min, both formulations start similarly, but Metformin alone shows quicker dissolution. Mid Phase 30–45 min noticeable difference: Metformin alone releases ~60% by 30 min, while the combination is closer to ~50%. Suggests papaya extract may delay drug release. Later Phase 60 - 75 min Metformin alone: ~90% - 95% release. Metformin with Papaya extract: ~85% - 88% release. The control group demonstrated consistent linearity in absorbance, reflecting stable dissolution. In contrast, the test group exhibited a non-linear, fluctuating absorbance pattern, suggesting potential interaction between the extract and metformin affecting dissolution kinetics or UV absorbance behavior. (Abdelgawad, n.d.) [10] (Figure 3).
Interpretation
Control group: Exhibits a predictable and smooth drug release profile.
Test group: Indicates that Carica papaya extract may interfere with metformin release or measurement, potentially affecting bioavailability.
Cumulative drug release (%): Metformin alone demonstrated a consistently higher release rate than the combination group across all time points.
AUC₀–₇₅ (%·min): The total drug exposure was higher for metformin alone (4582.5) compared to metformin with papaya extract (4155.0), indicating a reduction in bioavailable drug when the herbal extract is present.
Cmax: A slight reduction in maximum release (from 95% to 90%) was observed with the herbal extract, though Tmax remained unchanged.
Mean Residence Time (MRT): Increased from 46.44 min to 47.03 min with papaya extract, suggesting a mild delay in drug release.
Mean Concentration (C̄): Also reduced with papaya co-administration (61.10% vs. 55.40%), supporting the hypothesis of interference in drug solubilization or absorption kinetics.
The dissolution curve was found to be non-linear, exhibiting an initial quasi-linear release followed by a plateau, more pronounced in the herbal co-administration group.
References
- Młynarska E, Czarnik W, Dzieża N, Jędraszak W, Majchrowicz G, Prusinowski F, et al. Type 2 diabetes mellitus: new pathogenetic mechanisms, treatment and the most important complications. Int J Mol Sci. 2025;26(3):1094. Available from: https://doi.org/10.3390/ijms26031094
- Dutka M, Bobiński R, Ulman-Włodarz I, Hajduga M, Bujok J, Pająk C, et al. Sodium-glucose cotransporter 2 inhibitors: mechanisms of action in heart failure. Heart Fail Rev. 2021;26(3):603–22. Available from: https://link.springer.com/article/10.1007/s10741-020-10041-1
- Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–9. Available from: https://www.nejm.org/doi/10.1056/NEJM199602293340906
- Kazeem MI, Adeyemi AA, Adenowo AF, Akinsanya MA. Carica papaya Linn. fruit extract inhibited the activities of aldose reductase and sorbitol dehydrogenase: possible mechanism for amelioration of diabetic complications. Future J Pharm Sci. 2020;6(1):41. Available from: https://fjps.springeropen.com/articles/10.1186/s43094-020-00118-x
- Bagyalakshmi J, Selvakumaran SS, Priya K, Swathika R. Green synthesis and characterization of silver nanoparticles using Cassia fistula and assessment of its in vitro antidiabetic activity. Glob J Allergy. 2023;9(1):11. Available from: https://www.medsciencegroup.us/articles/Allergy-9-126.php
- Kumarasinghe HS, Kim JH, Kim SL, Kim KC, Perera RMTD, Kim SC, et al. Bioactive constituents from Carica papaya fruit: implications for drug discovery and pharmacological applications. Biomolecules. 2023;13(5):780. Available from: https://doi.org/10.3390/biom13050780
- Agada R, Thagriki D, Lydia DE, Khusro A, Alkahtani J, Al Shaqha MM, et al. Antioxidant and anti-diabetic activities of bioactive fractions of Carica papaya seeds extract. J King Saud Univ Sci. 2021;33(2):101342. Available from: https://jksus.org/antioxidant-and-anti-diabetic-activities-of-bioactive-fractions-of-carica-papaya-seeds-extract/
- Koul B, Pudhuvai B, Sharma C, Kumar A, Sharma V, Yadav D, et al. Carica papaya L.: a tropical fruit with benefits beyond the tropics. Diversity. 2022;14(8):683. Available from: https://www.mdpi.com/1424-2818/14/8/683
- Janardhanan B, Bavya C. Preparation, characterization, and pharmacokinetic interactions study of green synthesized silver nanoparticles of Pterocarpus marsupium with antidiabetic drug. Int J Res Pharma Pharm Sci. 2023;3(1):1–8. Available from: https://www.researchgate.net/publication/385598025_Preparation_Characterization_And_Pharmacokinetic_Interactions_Study_of_Green_Synthesized_Silver_Nanoparticles_of_Pterocarpus_Marsupium_with_Antidiabetic_Drug
- Abdelgawad MA, Elmowafy M, Musa A, Al-Sanea MM, Nayl AA, Ghoneim MM, et al. Development and greenness assessment of HPLC method for studying the pharmacokinetics of co-administered metformin and papaya extract. Molecules. 2022;27(2):375. Available from: https://www.mdpi.com/1420-3049/27/2/375
- Bagyalakshmi J, Bavya C. Challenges encountered in dissolution testing: the development of various dosage forms. Arch Pharmacol Pharmacol Res. 2022;3(2):1–7. Available from: https://irispublishers.com/appr/fulltext/Challenges-Encountered-in-Dissolution-Testing-the-Development-of-Various-Dosage-Forms.ID.000556.php
- Metry M, Shu Y, Abrahamsson B, Cristofoletti R, Dressman JB, Groot DW, et al. Biowaiver monographs for immediate release solid oral dosage forms: Metformin hydrochloride. J Pharm Sci. 2017;106(7):1727–36. Available from: https://jpharmsci.org/article/S0022-3549(17)30083-7/abstract
- Gupta RC, Chang D, Nammi S, Bensoussan A, Bilinski K, Roufogalis BD. Interactions between antidiabetic drugs and herbs: an overview of mechanisms of action and clinical implications. Diabetol Metab Syndr. 2017;9:59. Available from: https://dmsjournal.biomedcentral.com/articles/10.1186/s13098-017-0254-9
- Zhou Z, Wang C, Li M, Lan Q, Yu C, Yu G, et al. In vitro dissolution and in vivo bioequivalence evaluation of two metformin extended-release tablets. Clin Pharmacol Drug Dev. 2020;10(4):414–9. Available from: https://accp1.onlinelibrary.wiley.com/doi/10.1002/cpdd.857
- Fakeye TO, Oladipupo T, Showande O, Ogunremi Y. Effects of co-administration of extract of Carica papaya Linn (family Caricaceae) on the activity of two oral hypoglycemic agents. Trop J Pharm Res. 2007;6(1):671–8. Available from: https://www.ajol.info/index.php/tjpr/article/view/14645
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
