Archives of Community Medicine and Public Health
Technology transfer model for the formulation stage in biological products for public health
Jorge L Magalhães1,2*, Alessandra L Viçosa3 and Giselle Ribeiro de Barros4
2Global Health and Tropical Medicine, GHTM, Instituto de Higiene e Medicina Tropical, IHMT, Universidade Nova de Lisboa, UNL, Lisboa, Portugal.
3Department of Galenic Innovation, Deputy Direction of Education, Research and Innovation, Institute of Drug Technology, Oswaldo Cruz Foundation, Rio de Janeiro, RJ, Brazil
4Final Processing Department, Biomanguinhos, Oswaldo Cruz Foundation, Rio de Janeiro, RJ, Brazil
Cite this as
Magalhães JL, Viçosa AL, De Barros GR (2020) Technology transfer model for the formulation stage in biological products for public health Arch Community Med Public Health 6(1): 084-087. DOI: 10.17352/2455-5479.000084Technology transfer is a tool used to absorb knowledge and technology, in addition to increasing a country’s technological innovation. In the health sector, it is no different, especially in times of outbreaks, epidemics and global pandemic. Therefore, tools that assist in the creation of public policies contribute to the research, development and innovation of new technologies to produce vaccines and medicines. Thus, it proposes a universal technology transfer model based on International guides from the World Health Organization, Parenteral Drugs Association and the International Society of Pharmaceutical Engineering. It was concluded that the model ensures reliability between the parties and promotes a dynamic and rapid methodology for application in public health in the country.
Introduction
The pharmaceutical industry generates approximately US $ 1.3 trillion in drug production. It invests hard in research and development, innovation, marketing and distribution of medicines, which run through serums, vaccines and medicines in order to treat public health problems [1]. In this process, several technologies are used and, in order to accelerate business and innovations, pharmaceutical companies use technology transfer (TT) as a process to strengthen the development of new drugs [2,3]. TT is defined as “a logical procedure that controls the transfer of any product along with its documentation and professional expertise between development and manufacturing or between manufacturing sites” [4]. According to Mohite and Sangle [3], it has two sources of financing: the public sector (including universities) and the private sector [3]. Immunobiologicals have a great impact on prophylaxis and reduced mortality in diseases caused by infection, especially for the needy population who do not have access to health services. Thus, TT is one of the strategies for a country to have access to technology to produce these drugs more quickly to serve the population [4,5]. In this way, this work allowed us to propose a model in order to subsidize evenly a TT methodology for the formulation of production processes.
Methods
The World Health Organization (WHO), Parenteral Drugs Association (PDA) and the International Society of Pharmaceutical Engineering (ISPE) manuals were compared; WHO and PDA being selected. Experts (decision makers and technicians) from a Brazilian pharmaceutical company were unified and, in parallel, heard (authorization by the Brazilian Research Ethics Committee, opinion number 3,272,783).
Results and Discussions
According to WHO (2011) 80% of the world population does not have access to essential medicines. In this way, TT can become an excellent tool for pharmaceutical companies in developing or less developed countries, for example [6,7].
In the pharmaceutical industry, TT is used in processes such as the discovery and development of new drugs, from the beginning of the product’s life cycle to large-scale commercialization. In addition, some of its objectives are to transfer the product within and between companies and the knowledge of the process, used as a basis for manufacturing, analytical tests, process validation and continuous improvement [3,8]. It is worth mentioning that TT serves to assist in the transition of the medicine from the research and development stage to the marketing stage, identifying the important information during this process and clarifying doubts in the TT of products for different manufacturing locations [3].
According to WHO [4], TT is a procedure that must follow pre-determined steps in order to transfer knowledge and previously acquired experience so that it can be developed or marketed by another company [4]. Technologies are transferred through different forms such as licensing, franchising, turn-key, joint ventures, subcontracting, scientific cooperation, among others [9]. The technology transfer project (PTT) in the pharmaceutical industry consists of planned and controlled actions based on well-defined acceptance criteria. They must be used in the manufacturing process, analytical method, packaging component or any other step or process throughout the life cycle of the drug from a place of origin [10]. The management of a TT requires the creation of a multidisciplinary team that must be supervised so that the execution of the transfer activities and the routine activities are accomplished without any type of damage occurring in both. Another important point to be considered is the interaction between the teams of the two companies, which must overcome problems such as language and time zone [10]. According to [11], understanding workflows in companies is a pressing need and process modeling is the best option for this understanding, allowing companies to improve their processes, be more efficient, flexible, present competitive advantages and improve their services for customers and society [11-13].
Table 1 shows a summary of the recommendations expressed in each studied guide. Subsequently, to interviews with experts, it was possible to harmonize the results and propose a practical model for TT.
Final considerations
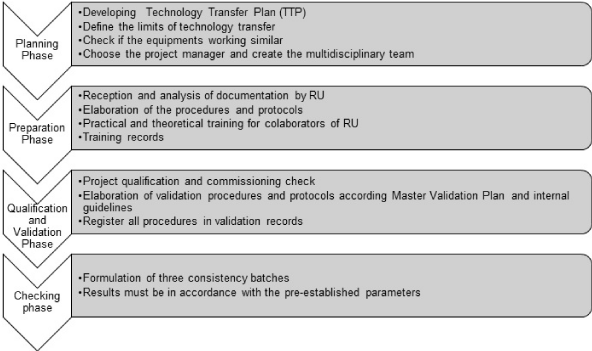
• Thus, after compiling the result of the interviews and standardized in the light of the comparison of the TT guides, the 4-phase model is proposed: Planning phase, preparation phase, qualification and validation phase and verification phase:Planning phase: SC and the RC must prepare a technology transfer plan that will guide the entire project and define the strategic approach with quality and risk management aspects. Define level and detail of the shared information. Verify that the facilities and equipment of the two units operate according to similar operating principles. The team must be quality assurance, quality control, production, regulatory affairs, engineering, finance, environmental management, health and safety, research and development and issues legal entities.
• Preparation phase: SC documentation is received and analyzed to carry out the process in the RC. Here the whole team is trained. Fortnightly follow-up meetings are established for the two companies. Minimum documentation: Product CQA, impurity profile, specifications (at least for APIcomponents / products and packaging), critical and non-critical process parameters, along with acceptable and proven ranges and ranges, manufacturing instructions, activity procedures related to the process, raw materials and auxiliaries, cleaning procedures, stability data available, validation documents (at least aseptic process and pathogen purification validation reports), process development documents (e.g. main technical reports and historical reports of the development process), previous regulatory record, manufacturing flow and process instructions, analytical methods and procedures, development report.
• Qualification and validation phase: obtained the master validation plan to carry out the project qualification, commissioning, installation qualification, operational qualification, performance qualification and process validation.
• Verification phase: manufacture 03 batches and these must demonstrate that the process parameters are under established control.
Figure 1 shows the synthesis of the 4 phases of the proposed model.
It can be concluded that the comparison of the technology transfer guides, added to the interviews carried out, provided subsidies to propose a harmonized model for better management and decision making. Thus, new information is included, such as the description of the implementation phases, the definition of the responsibilities of the participants in each step, control of changes and definition of responsibilities.
Ethics approval and consent to participate
Ethical approval was obtained from the institutional review board of INI/FIOCRUZ, final opinion number 3.353.006. Data were kept confidential and anonymous.
Funding
The project was partially funded by Biomanguinhos/FIOCRUZ.
Authors’ contributions
GRB participates in the beginning, designing, collecting data and writing the manuscript. JLM and AL guided, analyzed and revised all stages of the manuscript. JLM is the corresponding author of the manuscript.
Special thanks to Biomanguinhos/FIOCRUZ for this research, Health facilities, and for Professional Postgraduate Program in Management, Research and Development at Farmanguinhos/FIOCRUZ co-operation to be conducted the research.
- IQVIA (2018) Channel Dynamics Global Reference 2018. Link: https://bit.ly/2Xuzqmr
- Magalhães JL (2011) Laboratórios farmacêuticos oficiais e sua relevância para saúde pública do Brasil. Reciis 5: 85–99. Link: https://bit.ly/2ywHASL
- Mohite PB, Sangle SV, Bhusan B, Nautiyal U, Kumar S, et al. (2017) Technology transfer in Pharmaceutical Industry: A review. International Journal of Advances in Pharmaceutics 6: 1–7.
- WHO (2011) Annex 7 WHO guidelines on transfer of technology. WHO Technical Report 961: 285–309. Link: https://bit.ly/2Zz4C6J
- Brekke OH, Sandlie I (2003) Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov 2: 52–62. Link: https://bit.ly/2AWfcKE
- Moon S, Bermudez J, ’t Hoen E (2012) Innovation and Access to Medicines for Neglected Populations: Could a Treaty Address a Broken Pharmaceutical R&D System? PLoS Med 9: e1001218. Link: https://bit.ly/3ekCh8q
- World Health Organization (2016) WHO | Sixty-ninth World Health Assembly. Switzerland. Link: https://bit.ly/3gq1G22
- Alam MS, Ahmad J (2013) Pharmaceutical Technology Transfer: An Overview. International Journal of Pharmaceutical Sciences and Research IJPSR 4: 2441–2449. Link: https://bit.ly/36sTyJO
- Kumar V, Kumar U, Persaud A (1999) Building technological capability through importing technology: The case of Indonesian manufacturing industry. Journal of Technology Transfer 24: 81-96. Link: https://bit.ly/2TGRg4F
- PDA (2014) Technical Report No 65 Technology Transfer. 1. Link: https://bit.ly/2A5h5Eu
- Campos ALN (2014) Modelagem de Processos com BPMN (Brasport, Org.; 2a edição).
- Magalhães J, Hartz Z, Temido M, Antunes A (2018) Gestão do conhecimento em tempos de big data: um olhar dos desafios para os sistemas de saúde. Anais 17. Link: https://bit.ly/3gmWigm
- HO PL, Miyaji EN, Oliveira MLS, Dias WO, Kubrusly FS, et al. (2011) Economical value of vaccines for the developing countries--the case of Instituto Butantan, a public institution in Brazil. Link: https://bit.ly/3d2yV9D
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
