Global Journal of Rare Diseases
Mitochondrial disease, hypertrophic cardiomyopathy and cutaneous lupus in an infant with food hypersensitivity
E Estrada-Reyes1*, D Lopez-Gallegos2, E Faugier-Fuentes3, M Pardo-Castañeda4, E Barragán- Perez3, I Nuñez-Barrera3, M Sanchez-Ruiz1, S Nuñez-Barrera5, G Ramon-Garcia6 and Garcia-Beristain JC3
2Hospital Infantil Privado, Mexico City, Mexico
3Hospital Infantil de México “Federico Gómez”, Mexico City, Mexico
4Hospital ABC, Santa Fe, Mexico City, Mexico
5Hospital Centro Médico Dalinde, Mexico City, Mexico
6Hospital Infantil de México “Federico Gómez”. Mexico City, Mexico
Cite this as
Reyes EE, Gallegos DL, Fuentes EF, Castañeda MP, Perez EB, et al. (2020) Mitochondrial disease, hypertrophic cardiomyopathy and cutaneous lupus in an infant with food hypersensitivity. Glob J Rare Dis 5(1): 030-035. DOI: 10.17352/2640-7876.000027This paper describes a 4 month-old-girl with food allergies, mitochondrial disease, cutaneous lupus, and hypertrophic cardiomyopathy. She suffered from infectious pericarditis due to Coxsakie virus as a complication. She additionally presented bicytopenia (hemoglobin levels 8.9 g/dL, platelets 127 x 103 / µL), high lactate levels (5mmol/L), seizures, hypertrophic cardiomyopathy. This was suggestive of mitochondrial disease. Specific IgE levels were positive for milk, patch tests were positive for milk and soy, with a persistency of symptoms with extensively hydrolyzed formula as well as an amino acid-based formula. A chicken-based formula was well tolerated. Coenzyme Q10 levels were low (0.29L). Low Coenzyme Q10 levels in mitochondrial patients have been found as well as in allergic diseases. We suggest that food hypersensitivity should be ruled out in mitochondrial patients with low Coenzyme Q10 levels.
Introduction
Food allergies have dramatically increased although the reasons are unclear. In the developed world, this prevalence is approaching 10%. Many food allergies which start in early childhood are outgrown later in childhood [1]. Dermatologic and systemic manifestations of a food allergy are well recognized but we should not forget digestive tract manifestations [2]. Food hypersensitivity is a complex immune-mediated disease consisting of two mechanisms, IgE mediated and non IgE mediated [3].
Mitochondrial diseases are a group of genetic disorders that are characterized by defects in oxidative phosphorylation that encode structural mitochondrial proteins or proteins involved in mitochondrial function [4,5]. These are characterized by a wide variety of symptoms, such as: musculoskeletal and neurological symptoms, sensorial organs symptoms (sight and hearing disorders), organ and system damage: heart (cardiomyopathy or conductive defects), liver (hepatomegaly or development of liver failure), kidney (tubular diseases), gastro intestinal (vomit, diarrhea), blood (anemia, pancytopenia), and damage to the endocrine system [6] (Table 1).
Coenzyme Q10 is a lipid that acts in the mitochondrial respiratory chain, regardless of the cause. The impairment of CoQ10 status can result in profound deficits to mitochondrial function [7]. There is strong evidence that suggests mitochondrial dysfunction and allergy [8].
In this report, we describe the case of an infant who had a combination of food allergies, mitochondrial disease, cutaneous lupus, and Coenzyme Q10 low levels.
Case description
The patient was a 4 month-old-girl with a family history of atopy, and a sibling with atopic dermatitis and food allergies. Her mother suffers from Sjögren syndrome and hypothyroidism. During pregnancy, the mother suffered with a threatened miscarriage. She was delivered by cesarean section at 40 weeks of gestation, the baby was born with a body weight 2.59 kg, and a height of 49cm, with a screening showing normal metabolic and hearing. Hidden bottle with milk formula. The Umbilical cord dropped after 2 weeks. The mother tried to breastfeed but was unsuccessful due to baby reflux events.
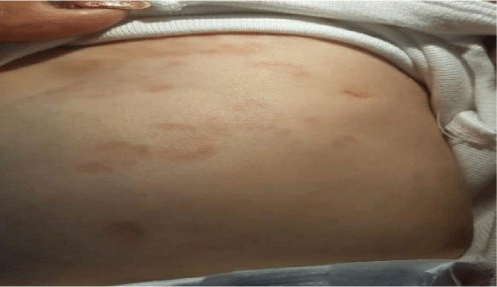
In the first few days after birth, the patient presented the following symptoms. She suffered from gastroesophageal reflux, diarrhea, and blood in her stools. Physical examination revealed skin with red lesions in a ring shape on the face, scalp, chest, and abdomen (Figure 1).
The patient was referred to Hospital Angeles Metropolitano at the age of 3 months. The growth retardation was evident, with a weight of 3360gms (<3) and body length of 51.1 (<3) percentiles. A delay on neurological development was observed. Her skin, with red lesions in a ring shape were displayed on her face, thorax and abdomen. ENT exploration was normal, with grade 3-4, systolic murmur, louder over the second right intercostal space, no clicks were heard. Respiratory sounds were normal, hepatic border at 3cm. She reacted to any milk formula even with extensively hydrolyzed formula and amino acid-based formulas. She presented vomit, diarrhea and blood in her stools.
The peripheral blood count with bicytopenia: hemoglobin level 8.9 g/dl, platelets count 127 x103/ µL, leukocytes count 8.00 x 103/ µL, neutrophils 0.6 x103 µL, lymphocytes 7.1 x 103/ µL. Serum immunoglobulin levels: IgA total 43. IgG 550, IgM 28, were IgG subtypes: IgG1 388 mg/dL, IgG2 40 mg/dL, IgG3 22 mg/dL, IgG4 1 mg/dL, IgA subtypes: IgA1 36.5 mg/dL, IgA2 4.6 mg/dL. IgG titles antibodies against relevant S, pneumoniae serotypes were normal. Flow cytometry of peripheral blood, lymphocyte subpopulation showed the following values: CD3 59% (reference range 54-76%), CD4 44% (36-55%), CD8 15% (12.24%), CD4/CD8 ratio 2.9 (1.7-3.9), CD19 36% (17-36). The liver, kidney, thyroid, and serum electrolytes function tests were normal as was an abdominal ultrasound.
A radioallergosorbent test (ImmunoCAP, Pharmacia, AB, Uppsala, Sweden) gave the following results> 75 IU/L for milk. We performed an atopy patch test: milk positive (13 p) soy (20 p) (Heine R Score) [9]. Due to a soy and milk allergy, we started a chicken-based formula which showed good tolerance, less vomit, diarrhea and blood in the stools. Her karyotype was normal. (Table 2)
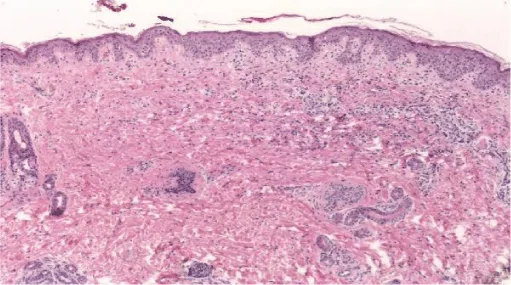
A skin biopsy reported: perivascular and interstitial vacuolar dermatitis, which is suggestive of cutaneous lupus (Figure 2). Antibodies anti-Sjögren, anticardiolipin, anti-DNA, and ANA were negative. However, anti-Ro was positive. At the same time, we ruled out the same antibodies in her mother.
Cardiologic evaluation showed the following features: Electrocardiogram with short P-R, heart rate 150x´, repolarization disorders in AVF, DII, IV suggestive of anterior and lower face ischemia.
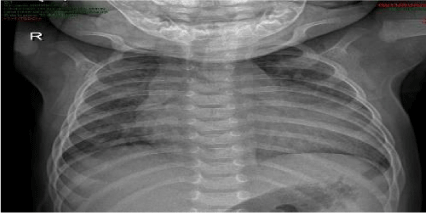
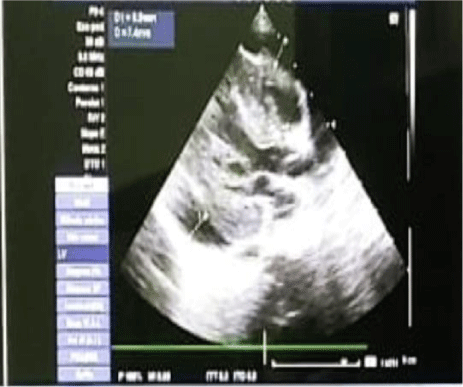
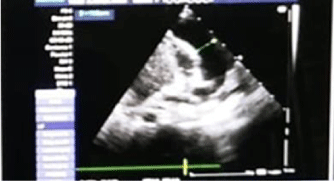
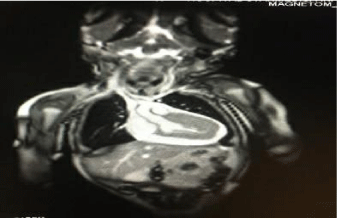
Chest radiography showed abdominal and bronchial situs with ICT 0.75, due to left ventricle growth, left arch and interstitial infiltrate (Figure 3). Echocardiogram reported: ejection fraction 87%, with severe concentric left ventricular hypertrophy with diastolic dysfunction consistent with hypertrophic cardiomyopathy. Interventricular septum and posterior wall of 8mm with score Z+5, indexed mass body surface 108 (Figure 4). With pericardial effusion without hemodynamic repercussion. Magnetic resonance reported acute myocarditis, left ventricular hypertrophy of the walls, papillary muscles and trabeculation increased, moderate pericardial effusion (Figure 5).
During her hospitalization, she presented two events of seizures that required a neurologic evaluation without any electroencephalographic and resonance feature. The evaluation concluded neurological delay.
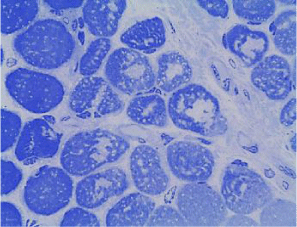
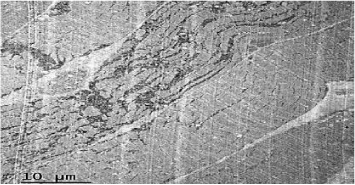
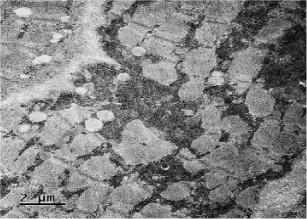
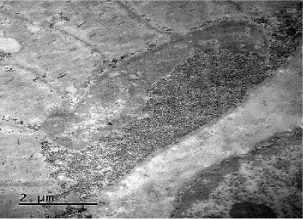
Coenzyme Q levels 0.29 mg/L, lactate levels 5mmol/L, pyruvate levels 0.4mg/dL, ratio lactate/pyruvate 12.5, muscle biopsy reported normal microscopy: some fibers with vacuoles in the sarcoplasm, Electronic microscopy: structure alteration of myofibrils with abundant subsarcolemmal and intrasarcolemmal glycogen deposits (Figures 6-9).
She presented torpid evolution of pericardial effusion, with management of the infectious event, and cardiac failure that required immunoglobulin administration and three boluses of methylprednisolone and antibiotic. An increase in the pericardial effusion with hemodynamic repercussion. A pericardial puncture was performed with an extraction of 90 cc. The following 8 hours lactate levels increased to 12.5 mmol/L, with metabolic acidosis and hyperlactatemia (20 mmol/L), with vasopressors treatment, torpid evolution without response to basic and advanced maneuvers. Positive Viral panel to Coxsakie B2 Ab 1:16, B3 AB 1:16. Quantiferon-TB gold: negative.
Discussion
This child underwent an extensive clinical and laboratory examination to establish an association between food allergies, mitochondrial diseases and low levels of Coenzyme Q10. Although we must not forget the onset of dermatitis (cutaneous lupus) since the first days of life (10); differential diagnosis of skin disorders in the newborn were considered, particularly atopic dermatitis which is highly associated with food allergies. However, the topography and morphology of the skin lesions are different in atopic dermatitis. These can take many forms (papules, vesicles, plaques, nodules and excoriations) [11].
Mitochondrial diseases have a wide spectrum of symptoms; the first systems affected are the muscular and nervous systems, and later, organs show symptoms of damage (Table 1). In the case we have described, the child presented neurological delay and seizures as nervous system damage, followed by cardiomyopathy, gastrointestinal symptoms and bicytopenia, and growth delay due to the damage in organs and systems [5,6].
Although it is described “phenomena of irregular red fibers” as a main marker of mitochondrial diseases, there are other morphologic characteristics in mitochondrial diseases, such as: the significant increase in the size of mitochondria, accumulation of glycogen, lipids and calcium conglomerates in the subsarcolemmal, and a decrease in the activity of mitochondrial enzymes. This is the biopsy description of our patient. The phenomenon of irregular red fibers can develop in other diseases such as: Duchene myodystrophy, dermatomyositis or by drugs such as clofibrate [6,12,13]. Compelling evidence has recently suggested that mitochondria in mammals, have multiple roles in immunity, and that in addition to being the powerhouse of the cell, they also represent the powerhouse of immunity [14]. In this light, increasing evidence strongly suggests a relevant role for mitochondrial dysfunction, and consequent excessive generation of reactive oxygen species (ROS) in an allergy. Mitochondrial dysfunction and elevated ROS have been reported in atopic dermatitis, allergic rhinitis and asthma [15]. Studies in a murine model suggest that the liver could act as a source of CD4+T cells and could play an important role in the IgE response to dietary antigens [8]. These features could link the symptoms and evolution in the patient.
The importance of Coenzyme Q10 (CoQ10) in mitochondrial function is widely recognized. The significance of CoQ10 deficiency has only recently been considered to have clinical importance. Low levels of CoQ10 in plasma and serum have been reported in numerous disorders, including asthma. Miles reported CoQ10 pathologic, and mitochondrial respiratory chain abnormalities in a group of children with eosinophilic gastrointestinal disease and food intolerance. He concluded that children with recurrent food intolerance and allergies may acquire CoQ10 deficiency with disease progression [16].
The prevalence of food allergies may vary from 0.3% to 10% worldwide and milk formulas have high allergenic potential for infants. We can choose extensively hydrolyzed formulas or amino acid-based formulas, but there are more culprit proteins such as soy, corn, wheat, and egg. In the patient our choice was a chicken-based formula. Patients with food allergies may have gastrointestinal, respiratory, and cutaneous symptoms. In our patient, the food allergy background led us to suspect this [17,18].
Conclusion
Patients with mitochondrial disease suspicion, Coenzyme Q 10 low levels should be evaluated as well for food hypersensitivity. In order to treat them with early CoQ10 supplementation and it can result in clinical improvement in conjunction with an avoidance diet. A number of disorders associated with CoQ10 deficiency have been successfully treated via CoQ10 supplementation [19]. CoQ10 has a central role in the metabolism of all cells, and its deficiency is linked to the pathogenesis of a range of disorders. Primary CoQ10 deficiency results from genetic defects in the multi-step CoQ10 biosynthetic pathway. Brain, muscle and kidney tissues are particularly susceptible to the metabolic consequences of a deficit in the status of this isoprenoid, presenting clinically with disorders such as ataxia, myopathy and nephrotic syndrome, respectively. Early identification in CoQ10 deficiency is essential, since patients may show remarkable clinical improvement following CoQ10 supplementation when administered at an early stage of disease [19-22].
- John SJ (2015) Food allergy Epidemiology and natural history. Immunol Allergy Clin N Am 35: 45-59. Link: https://bit.ly/3eplJgO
- Boonyaviwat O, Punchama P, Jirapongsanannuruk O, Vichyanond P, Visitsunthorn N (2015) Role of atopy patch test for diagnosis of food allergy-related gastrointestinal symptoms in children. Pediatr Allergy Immunol 26: 737-741 Link: https://bit.ly/3ej3TMm
- Ashley S, Dang T, Kopiln J, Martino D, Prescott S (2015) Food for thought: progress in understanding the causes and mechanisms of food allergy. Curr Opin Allergy Clin Immunol 15: 237-242. Link: https://bit.ly/3enSqed
- Gorman GS, Chinnery PF, Di Mauro S, Hirano M, Koga Y, et al. (2016) Mitochondrial diseases. Nat Rev Dis Primers 2: 16080. Link: https://bit.ly/2HR8jhP
- Cohen HB (2019) Mitochondrial and Metabolic Myopaties. Continuum 25: 1732-1766. Link: https://bit.ly/3kWw5Hs
- Portnov A (2018) Sintomas de enfermedades mitocondriales. Link: https://bit.ly/3oR9Q7U
- Yubero D, Allen G, Artuch R, Montero R (2017) The value of Coenzyme Q10 determination in Mitochondrial Patients. J Clin Med 37: 2-10. Link: https://bit.ly/2HPuLYD
- Trinchese G, Papero L, Aitoro R, Fierro C, Varchetta M, et al. (2018) Hepatic mitochondrial dysfunction and immune response in a murine model of peanut allergy. Nutrients 10: 744: 2-12. Link: https://bit.ly/2HWzY0f
- Heine GR, Verstege A, Mehl A, Staden U, Rolinck-Werninhaus, et al. (2006) Proposal for a standardized interpretation of the atopy patch test in children with atopic dermatitis and suspected food allergy. Pediatr Allergy Immunol 17: 213–217. Link: https://bit.ly/38jCzg7
- Aguilera-Peyro P, Vicente-Villa A, Gonzalez-Enseñat MA (2011) Lupus eritematoso neonatal. Semin Fund Esp Reumatol 12: 15-20.
- Mihm CM, Soter AN, Dvorak FH, Austen MD (1976) The structure of normal skin and morphology of Atopic Eczema. Journal of Investigative Dermatology 67: 305-312. Link: https://bit.ly/3jT8HZY
- Ridaura-Sanz (2008) Biopsia muscular. Acta Pediatr Mex 29: 347-354. Link: https://bit.ly/34Pm2yb
- Rosero-Salazar DH, Salazar-Monsalve L, Tovar-Sánchez MA (2014) Histoquímica enzimática en el diagnóstico de miopatías: revisión sistemática. Medicina & Laboratorio 20: 467-488. Link: https://bit.ly/2TQtDq0
- Mills LE, Kelly B, O´Neill AJL (2017) Mitochondria are the powerhouses of immunity. Nat Immunol 18: 488-498. Link: https://bit.ly/3oMggFz
- Jang H, Kim M, Hong JY, Cho HJ, Kim CH, et al. (2020) Mitochondrial and nuclear mitochondrial variants in allergic diseases. Allergy. Asthma Immunol Res 12: 877-884. Link: https://bit.ly/2I3JqPi
- Miles VM, Putnam EP, Miles L, Tang HP, DeGrauw JA, et al. (2011) Acquired coenzyme Q10 deficiency in children with recurrent food intolerance and allergies. Mitochondrion 127-135. Link: https://bit.ly/3mKGs1q
- Vanderhoof J, Moore N, de Boissieu D (2016) Evaluation of an amino acid-based formula in an infant not responding to extensively hydrolyzed protein formula. J Pediatr Gastroenterol Nutr 63: 531-533. Link: https://bit.ly/2I32JIZ
- Jirapinyo P, Densupsoontorn N, Kangwanpornsiri C, Wongarn R (2012) Chicken-based formula is better tolerated than extensively hydrolyzed casein formula for the management of cow milk protein allergy in infants. Asia Pac J Clin Nutr 21: 209-214. Link: https://bit.ly/3oTxJvO
- Hargreaves I, Heaton AR, Mantle D (2020) Disorders of Humen Coenzyme Q10 Metabolism: An Overview. Int J Mol Sci 21: 1-13.
- Qunizii MC, DiMauro S (2007) Human Coenzyme Q10 Deficiency. Neurochem Res 32: 723-727. Link: https://bit.ly/3kSYdLt
- Hernandez-Camacho JD, Bernier M, Lopez-Lluch G, Navas P (2018) Coenzyme Q10 Supplementation in Aging and Disease. Front Physiol 44: 1-11.Link: https://bit.ly/2TLsVKu
- Garrido-Maraver A, Cordero DM, Oropesa-Avila M, Fernández Vega A, de la Mata M, et al. (2014) Coenzyme Q10 Therapy. Mol Syndromol 5: 187-197. Link: https://bit.ly/2HZVPEr

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
