Global Journal of Rare Diseases
Intrahepatic Cholangiocarcinoma Skin Metastasis in a Patient with Hidradenitis Suppurativa: A Rare Entity
Ali Y Fakhreddine1*, Theresa Yang2, Guanghong Liao3 and Bahman Chavoshan2,4,5
2Department of Medicine, Harbor UCLA Medical Center, Torrance, CA 90502, United States of America
3Department of Pathology, Harbor UCLA Medical Center, Torrance, CA 90502, United States of America
4Department of Medicine, St. Mary Medical Center, Long Beach, CA 90813, United States of America
5Department of Medicine, University of California, Los Angeles, CA 90095, United States of America
Cite this as
Fakhreddine AY, Yang T, Liao G, Chavoshan B (2020) Intrahepatic Cholangiocarcinoma Skin Metastasis in a Patient with Hidradenitis Suppurativa: A Rare Entity. Glob J Rare Dis 5(1): 010-014. DOI: 10.17352/2640-7876.000022We present a challenging diagnosis of disseminated intrahepatic cholangiocarcinoma presenting with perineal cutaneous masses in a young African American male with known hidradenitis suppurativa. The patient was a 39-year-old male who presented to the emergency department with difficulty walking due to severe gluteal swelling and pain. The patient had an 18-month history of biopsy-proven hidradenitis suppurativa. Examination under anesthesia disclosed a 9×6 cm left perianal mass and right 5×3 cm right peri-scrotal mass; both masses were excised and revealed adenocarcinoma with signet ring cell differentiation. The patient was diagnosed with widely metastatic adenocarcinoma of unknown primary. Initiation of palliative capecitabine was planned however the patient decompensated and expired one month from the time of his diagnosis due to septic shock. Autopsy with histologic staining revealed innumerable liver lesions consistent with rare variant Intrahepatic Cholangiocarcinoma (ICC) with signet-ring cell differentiation. Multiple red papules were noted on the aortic valve with histology similarly demonstrating signet-ring cell adenocarcinoma. Cutaneous metastasis of cholangiocarcinoma has been reported but is rare, and to our knowledge this is the first reported case of cholangiocarcinoma cutaneous metastasis in a patient with hidradenitis suppurativa. This case re-emphasizes the protean presentation and aggressive metastatic potential of ICC. It also highlights the importance of maintaining a wide differential for dermatologic lesions in patients with hidradenitis suppurativa and postulates a mechanism for metastatic seeding of areas affected by this disease.
Abbreviations
ICC: Intrahepatic cholangiocarcinoma; SCC: Squamous cell carcinoma; CT: Computed tomography.
Fakhreddine A, Yang T, Liao G, Chavoshan B. Intrahepatic Cholangiocarcinoma Skin Metastasis in a Patient with Hidradenitis Suppurativa: A Rare Entity
Introduction
Hidradenitis Suppurativa (HS) is a chronic, disabling disease of the apocrine glands. Prevalence rates have been estimated to be around 1%, increasing to up to 4% in certain subpopulations such as females in the 3rd to 4th decades of life [1,2]. The disease is commonly complicated by sinus fistulous tract formation and abscesses resulting in significant social and economic disability [1,3]. While infection is a common complication of HS, the primary lesions are believed to be due to dysregulated immunity and inflammation especially given significant clinical response to anti-TNF therapy [2,4]. Malignancy on the other hand is a rare complication of the natural history of disease with squamous cell carcinoma representing the most common complication of chronic, recurrent HS. Cutaneous adenocarcinoma is an even rarer complication with few case reports in the literature [3,5-7]. Therefore, a high index of suspicion is required to ensure malignant cutaneous lesions are not missed in patients with HS.
While Intrahepatic Cholangiocarcinoma (ICC) is notorious for an aggressive metastatic potential including cutaneous metastasis, this is the first case of ICC perianal metastases complicating pre-existent HS. The most common sites of cholangiocarcinoma metastasis include bone (25%), peritoneum (25%), lung (19%), portal lymph nodes, and portal and hepatic veins [8]. Cutaneous metastases in ICC are not uncommon as result of seeding following surgery or percutaneous biopsy. When it does occur beyond these sites, the scalp is the most common cutaneous site of distant metastasis described [9].
Prognosis of ICC with metastasis is poor however palliative chemotherapy can still increase survival by several months, emphasizing the utility of an expedient diagnosis [10]. Here we describe a challenging diagnosis of ICC in a young African American male presenting with worsening perineal lesions in the setting of hidradenitis suppurativa. We chose to present this case as it effectively demonstrates the importance of both considering adenocarcinoma in the differential of cutaneous lesions in HS and specifically metastatic ICC in the differential of poorly differentiated adenocarcinoma of unknown primary.
Case report
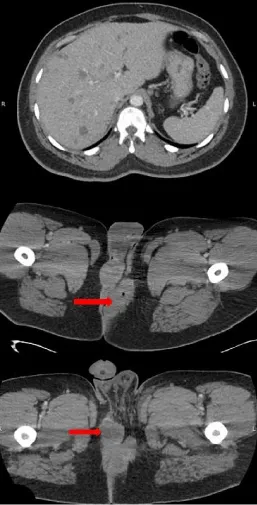
A 39-year-old obese African American male presented to the emergency department with difficulty walking due to severe gluteal swelling and pain. The patient had an 18-month history of biopsy-proven hidradenitis suppurativa previously managed with antibiotics and multiple incision and drainage procedures. CT of the chest, abdomen, and pelvis was remarkable for left perianal induration (8.3×3.7 cm in size) increased from prior imaging, as well as multiple new low-density lesions in the liver (Figure 1). Examination under anesthesia disclosed a 9×6 cm left perianal mass and a 5×3 cm right perineal mass; both were excised and sent for histological evaluation. Given the liver findings, the patient underwent upper endoscopy and colonoscopy which were unremarkable. CT-guided biopsy of presumed hepatic metastases was attempted but none of the hepatic lesions could be accessed at that time per radiology and the patient was discharged with outpatient plan for inguinal lymph node biopsy.
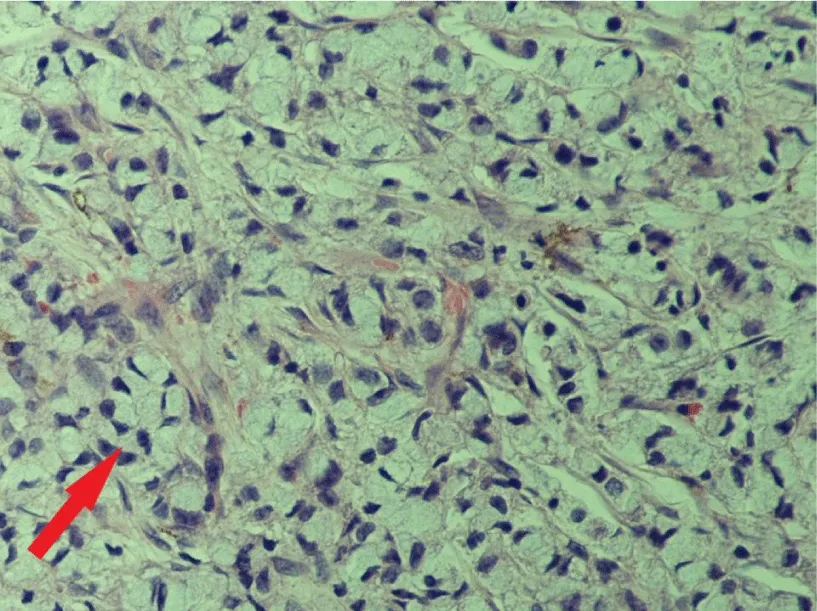
Only a few days following discharge the patient returned to the emergency department with complaint of urinary obstruction and suprapubic fullness. CT Pelvis demonstrated density invasion into the left corpus cavernosum. Bone marrow biopsy for worsening pancytopenia revealed near total replacement with metastatic adenocarcinoma with signet ring cell morphology. Histologic evaluation of the previously excised perineal masses revealed adenocarcinoma with signet ring cell differentiation (Figure 2). Immunohistochemical stains for cytokeratin 7 and cytokeratin 20 were positive. There was no evidence of abscess or infectious organisms.
The patient was presumptively diagnosed with metastatic adenocarcinoma of unknown primary. Initiation of palliative capecitabine was planned however the patient’s hospitalization was complicated by perianal bleeding in the setting of thrombocytopenia, sepsis presumed secondary to hospital acquired pneumonia, and delirium. The patient expired one month from the time of diagnosis due to asystole cardiac arrest with no chemotherapy given.
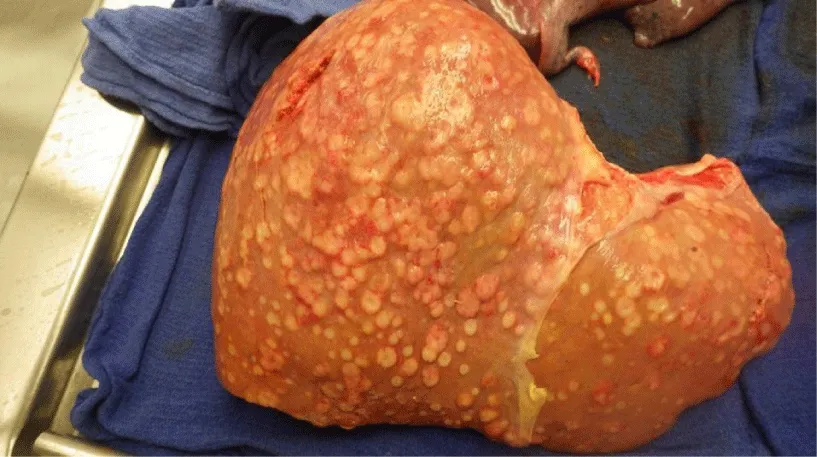
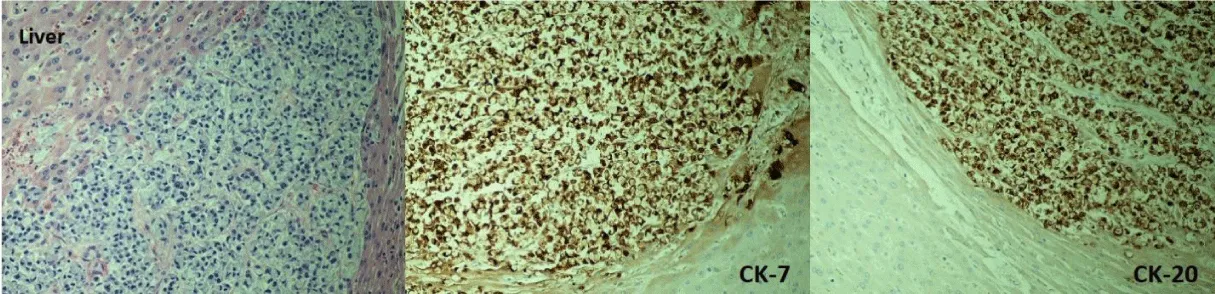
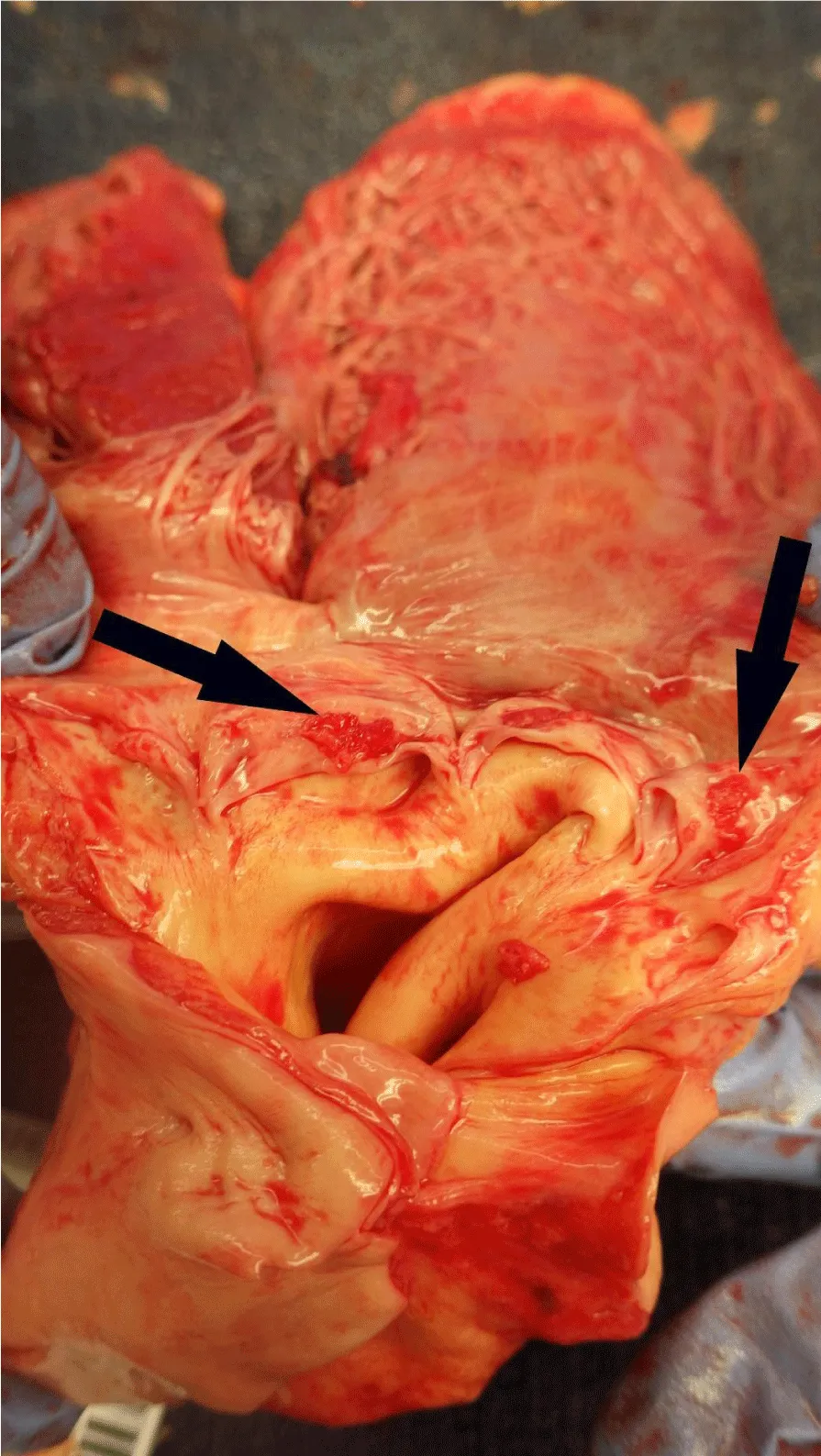
Post-mortem autopsy of the liver (Figure 3) revealed innumerable liver lesions with histology demonstrating adenocarcinoma with signet-ring cell differentiation (Figure 4). Staining was positive for CK7, CK20, CEA, BerEP4, EMA, and CK19, focally positive for CA19.9, and negative for HepPar and PSA. Multiple red papules were noted on the aortic valve (Figure 5) with histology demonstrating signet-ring cell adenocarcinoma. Additional sites of metastasis were noted in the epidural soft tissue around the lumbar spinal cord, the pericardiac fat, bone marrow, and multiple nodules in the perianal tissue. Malignancy burden was greatest in the liver with no other identifiable primary on necropsy. In combination with staining pattern, post-mortem diagnosis was rare variant signet ring cell intrahepatic cholangiocarcinoma with diffuse metastasis.
Discussion
The diagnosis of metastatic signet ring cell ICC described above was only made on autopsy. In addition to emphasizing the persistent diagnostic utility of autopsy in modern medicine, the case describes a rapid clinical deterioration that impresses the importance of an expedient diagnosis. A high index of suspicion for ICC should be maintained in all cases of carcinoma on unknown primary, including cutaneous adenocarcinoma metastasis in patients with HS.
Cholangiocarcinomas are the second most common primary liver malignancy and represent roughly 3% of cancers worldwide [10]. Whereas signet ring cell morphology of adenocarcinoma is not uncommon in luminal gastrointestinal malignancies, it is rarely described in non-gallbladder biliary cancer. A 2017 case report and review of the literature found only 12 total reported cases of primary signet ring cell cholangiocarcinoma and interestingly presented the only other case of diffusely metastatic presentation besides our patient [11].
ICC is less common than peri-hilar or extrahepatic cholangiocarcinoma but has had increasing incidence and mortality in the United States over the last three decades [10,12,13]. Three morphologic growth types of ICC are identified by the Liver Cancer Study Group of Japan, and each carry different spread and metastasis characteristics [14,15]. In general, intrahepatic spread predominantly occurs along the sinusoidal space. However vascular, lymphatic, and biliary duct invasion are also frequently evident histologically at time of diagnosis which corresponds with the aggressive metastatic nature of ICC [16]. Due to early presence of distant metastasis, only a third of patients tend to be candidate for surgical resection at time of diagnosis. Even in patients that undergo surgery with curative intent, 5 year survival rates remain at 14-40% [10,17].
The metastatic potential of ICC is due to an aggressive, well-described tumor microenvironment comprised of cancer-associated fibroblasts, cytokines, growth factors, and tumor-associated macrophages that drive the invasion-metastasis cascade and lymphangiogenesis and angiogenesis mechanisms necessary for successful implantation of distant secondary tumors [18,19]. The usual sites of distant metastasis described for ICC include the lung, peritoneum, bone, pancreas, and adrenals which corresponds with local lymphatic and vascular anatomy [20]. The presence of perineal and cardiac metastases in our patient however has never been previously described.
Cutaneous metastasis of internal organ malignancy in general is rather uncommon, occurring at a rate of 0.9-10% [21,22]. Even when accounting for under-reporting, cholangiocarcinoma likely comprises a fraction of a percentage of cutaneous metastases. A recent systematic review of the literature between 1978 and 2014 by Liu et al identified a total of 30 patients with cutaneous metastasis of cholangiocarcinoma [9]. Only 15 cases described distant metastasis not involving a prior surgical or drainage site. The scalp was the most common cutaneous metastatic site presumably due to abdominal vasculature communication with the valveless vertebral venous plexus. Chest and knee metastasis were also reported. Perineal metastases have not been described. In the context of our patient’s pre-existent chronic active perianal hidradenitis suppurativa we postulate the rarity of this finding may represent a consequence of the co-morbidity rather than a coincidence. TNF- has been demonstrated to drive invasion, migration, and angiogenesis in the cholangiocarcinoma tumor microenvironment [18,19]. As an inflammatory disease, TNF- serum levels are elevated and play a central role in the pathogenesis HS, even representing a therapeutic target [2]. Higher systemic and local levels of TNF- of pre-existing HS disease may therefore have not only contributed to the aggressive metastatic nature of the patient’s ICC but also increased the chances for successful implantation of a secondary tumor in the perineum when combined with a potential chemokine effect and increased blood circulation due to active inflammation.
Cardiac valve metastasis is even less common than cutaneous metastasis, and to our knowledge this is also the first reported case of aortic valve seeding by cholangiocarcinoma. Cardiac metastases occur with rates of 12%-15% seen in autopsy series of patient with disseminated cancer. Sites of metastasis overwhelmingly involve the epicardium and myocardium. Endocardial metastases are rare and occur in 5%-6% of cardiac metastasis. Rates of valvular seeding are poorly defined and described as rare in one autopsy series. 11 total cases of liver and biliary malignancy cardiac metastasis were reported at a rate of 2%-5% in various autopsy series. Location was not described [23]. The presence of cardiac metastasis in this patient in the absence of known valvulopathy may be due simply to the advanced stage of disease at diagnosis and aggressive variant of adenocarcinoma.
Finally, cutaneous malignancy in hidradenitis suppurativa is generally limited to Squamous Cell Carcinoma (SCC) either through transformation or through increased risk for SCC [24,25]. Cutaneous adenocarcinoma in patients with HS is rare with only a few other case reports in the literature [3,5-7]. Although ICC cutaneous metastasis in patients with hidradenitis has not been previously reported, HS patients are interestingly at an increased risk of primary liver malignancies [24].
In summary, this case describes an extremely rare histologic variant of ICC with a previously unreported metastatic pattern. The rare finding of perineal metastasis in the location of active HS suggests a possible interaction between the inflammatory pathophysiology of HS and the tumor microenvironment of cholangiocarcinoma which promoted successful secondary tumor implantation. The case highlights the importance of evaluating suspicious dermatologic lesions in HS and performing a full evaluation for an alternative site of primary malignancy in the case of malignant histology. It also serves an important reminder to always consider ICC on the differential for widely metastatic cancer of unknown primary.
Author Contributions
Fakhreddine A, Yang T, Liao G, and Chavoshan B designed the report; Fakhreddine A and Liao G collected the patient’s clinical data; Fakhreddine A, Liao G, Yang T, and Chavoshan B examined the data and wrote the paper.
Informed Consent statement
Waiver requested given patient deceased.
- Alikhan A, Lynch PJ, Eisen DB (2009) Hidradenitis suppurativa: A comprehensive review. J Am Acad Dermatol 60: 539-561. Link: https://bit.ly/3bujhTK
- Jemec GBE (2012) Hidradenitis suppurativa. N Engl J Med 366: 158-164.
- Anderson BB, Cadogan CAM, Gangadharam D (1982) Hidradenitis suppurativa of the perineum, scrotum, and gluteal area: presentation, complications, and treatment. J Natl Med Assoc 74: 999-1003. Link: https://bit.ly/2yxMsa6
- Kimball AB, Okun MM, Williams DA, Gottlieb AB, Papp KA, et al. (2016) Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med. 375: 422-434. Link: https://bit.ly/2VtQt8f
- Marti L, Nussbaumer P, Breitbach T, Hollinger A (2001) Perianal mucinous adenocarcinoma. A further reason for histological study of anal fistula or anorectal abscess. Chirurg 72: 573-577. Link: https://bit.ly/3axsg5n
- do Val ICC, Almeida Filho GL, Corrêa A, Neto N (2007) Chronic hidradenitis suppurativa and perianal mucinous adenocarcinoma. A case report. J Reprod Med 52: 100-102. Link: https://bit.ly/34XZGZB
- Kannan S, Choksi A (2014) A case of cutaneous metastatic adenocarcinoma mimicking lesions of hidradenitis suppurativa. J Am Acad Dermatol 70: AB113. Link: https://bit.ly/3bv9igV
- Kanel GC, Korula J (2011) Neoplasms and Related Lesions. In: Atlas of Liver Pathology. 3rd ed. Philadelphia: Saunders 249-321.
- Liu M, Liu BL, Liu B, Guo L, Wang Q, et al. (2015) Cutaneous metastasis of cholangiocarcinoma. World J Gastroenterol 21: 3066-3071. Link: https://bit.ly/2Kt7CJ6
- Benson AB, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR (2020) Hepatobiliary Cancers. National Comprehensive Cancer Network.
- Yonat S, Amir D, Menahem B, et al. (2017) An Unusual Case of Signet Ring Cell Cholangiocarcinoma- Case Report and a Review of Literature. Adv Res Gastroenterol Hepatol 6
- Welzel TM, McGlynn KA, Hsing AW, O’Brien TR, Pfeiffer RM (2006) Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 98: 873-875. Link: https://bit.ly/3axnVyW
- Zhang H, Yang T, Wu M, Shen F (2016) Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett 379: 198-205. Link: https://bit.ly/2xJxiP0
- Brown KM, Parmar AD, Geller DA (2014) Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am 23: 231-246. Link: https://bit.ly/2Y2bkkP
- Poultsides GA, Zhu AX, Choti MA, Pawlik TM (2010) Intrahepatic Cholangiocarcinoma. Surg Clin North Am 90: 817-837. Link: https://bit.ly/3eMe5ws
- Nakajima T, Kondo Y, Miyazaki M, Okui K (1988) A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma: Histologic classification and modes of spreading. Hum Pathol 19: 1228-1234. Link: https://bit.ly/2Kqnk7L
- Moeini A, Sia D, Bardeesy N, Mazzaferro V, Llovet JM (2016) Molecular Pathogenesis and Targeted Therapies for Intrahepatic Cholangiocarcinoma. Clin Cancer Res 22: 291-300. Link: https://bit.ly/2VRQt0N
- Roy S, Glaser S, Chakraborty S (2019) Inflammation and Progression of Cholangiocarcinoma: Role of Angiogenic and Lymphangiogenic Mechanisms. Front Med 6. Link: https://bit.ly/2Y9Ew9R
- Guan X (2015) Cancer metastases: Challenges and opportunities. Acta Pharm Sin B 5: 402-418. Link: https://bit.ly/2VLa6ru
- Baheti AD, Tirumani SH, Shinagare AB, Rosenthal MH, Hornick JL, et al. (2014) Correlation of CT patterns of primary intrahepatic cholangiocarcinoma at the time of presentation with the metastatic spread and clinical outcomes: retrospective study of 92 patients. Abdom Imaging 39: 1193-1201. Link: https://bit.ly/3axwlGA
- Hussein MRA (2010) Skin metastasis: a pathologist’s perspective. J Cutan Pathol 37: e1-e20. Link: https://bit.ly/2VRQfqt
- Lookingbill DP, Spangler N, Helm KF (1993) Cutaneous metastases in patients with metastatic carcinoma: A retrospective study of 4020 patients. J Am Acad Dermatol 29: 228-236. Link: https://bit.ly/34Xg38V
- Burke A, Virmani R (1996) Tumors of the Heart and Great Vessels. In: Atlas of Tumor Pathology. Washington, D.C.: Armed Forces Institute of Pathology; 1996:Third Series, Fascicle 16.
- Lapins J, Ye W, Nyrén O, Emtestam L (2001) Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol 137: 730-734. Link: https://bit.ly/2KpdkLR
- Huang C, Lai Z, He M, Zhai B, Zhou L, et al. (2017) Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa A case report and literature review. Med (United States) 96: e5857. Link: https://bit.ly/2xJwb1M

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
