Global Journal of Rare Diseases
Cardiocutaneous Syndrome: The Tale between Heart and Skin
A K M Monwarul Islam*, Amiruzzaman Khan and Zakir Hossain
Cite this as
Islam AKMM, Khan A, Hossain Z (2017) Cardiocutaneous Syndrome: The Tale between Heart and Skin. Glob J Rare Dis 2(1): 001-006. DOI: 10.17352/2640-7876.000005Cardiocutaneous syndromes are rare, genetically determined disorders in which arrhythmogenic cardiomyopathy is accompanied by characteristic cutaneous phenotypes of woolly hair and palmoplantar keratoderma. Pattern of cardiac involvement differs in different cardiocutaneous syndromes; right ventricle (RV) is predominantly affected in the form of classical arrhythmogenic right ventricular cardiomyopathy/ dysplasia (ARVC/D) in Naxos disease whereas the left ventricle (LV) is mainly involved in the form of dilated cardiomyopathy in Carvajal syndrome. Both conditions are transmitted in autosomal recessive manner, and results from mutations in cell adhesion molecules compromising the integrity of desmosomal junctions of skin and myocardium. Naxos disease usually presents by adolescence with malignant ventricular arrhythmia or cardiac arrest whereas Carvajal syndrome is manifested earlier with heart failure. Cardiomyopathy is diagnosed by the 2010 Task Force Criteria. Implantation of automated implantable cardioverter-defibrillator (AICD) forms the mainstays of treatment by preventing sudden cardiac death. Genetic testing is available. Several international databases and registries, often as a part of other genetically determined arrhythmogenic disorders, are directed to better characterize and manage these cardiocutaneous syndrome patients.
Abbreviations
3D: Three-Dimensional; AICD: Automated Implantable Cardioverter-Defibrillator; ARVC/D: Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia; CMR: Cardiac Magnetic Resonance: CT: Computed Tomography; ECG Electrocardiogram LBBB: Left Bundle Branch Block; LV: Left Ventricle; RBBB: Right Bundle Branch Block; RV: Right Ventricle; RVOT: Right Ventricular Outflow Tract; SAECG: Signal-Averaged ECG; TFC: Task Force Criteria; TTE: Transthoracic Echocardiography; VAD: Ventricular Assist Device; VT: Ventricular Tachycardia; WCD: Wearable Cardioverter-Defibrillators.
Introduction
Naxos disease and Carvajal syndrome are the 2 closely-related genetically determined conditions where inherited cardiomyopathy is accompanied by characteristic cutaneous phenotype of palmoplantar keratoderma and woolly hair [1]. The cutaneous manifestations are similar in 2 conditions, but the pattern of cardiomyopathy differs; in Naxos disease, right ventricle (RV) is predominantly affected, whereas in Carvajal syndrome, the cardiomyopathy mainly affects the left ventricle (LV). At present, they are considered as subtypes of arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D); majority of the ARVC/Ds are autosomal dominant disorders, whereas in Naxos disease and Carvajal syndrome, the ARVC/Ds are transmitted in a autosomal recessive manner [2-7]. These autosomal recessive forms of ARVC/D i.e. Naxos disease and Carvajal syndrome appear to have a more severe prognosis compared with the dominant form of ARVC/D.
Epidemiology
Naxos disease was first described by Protonotarios et al. in 4 families originating from the Greek island of Naxos [8]. Later, the disease was reported from other parts of the world including other Greek islands of Milos, Evia and Mykonos, Turkey, Israel, Saudi Arabia, India and Bangladesh [4,9-13]. Carvajal syndrome with predominant LV involvement has been described from India and Ecuador [14-16]. Biventricular involvement has also been described [17]. Naxos disease is prevalent in Greek islands, and the prevalence may be up to 1:1000 [18].
Pathophysiology
Genetics: ARVC/D is a genetic disease of clinical and genetic heterogeneity with a familial background consistent with an autosomal-dominant trait of inheritance in most cases; only a minority are inherited as autosomal recessive manner, either or not associated with palmoplantar keratoderma and woolly hair. ARVC/D-causing genes encode cardiac desmosomes in majority and non-desmosomal structures in minority, the latter predisposing to the same or an overlapping disease phenotype. Compound/digenic heterozygosity has been found in up to one-fourth of ARVC/D-causing desmosomal gene mutation carriers, in part explaining the phenotypic variability. Naxos disease and Carvajal syndrome result from genetic defects affecting the cell adhesion molecules plakoglobin and desmoplakin. Naxos disease is caused by deletion mutation in plakoglobin gene, whereas Carvajal syndrome results from mutations in the desmoplakin gene. For Naxos disease, Coonar et al. in 1998 mapped the genetic locus to 17q21 [19] and 2 years later, McKoy et al. reported a 2 base pair deletion in the plakoglobin gene (JUP) resulting in a frameshift and premature termination of the protein [5]. For Carvajal syndrome, first recognized by Rao et al. in 1996 in India [16] and then in several Ecuadorian families by Dr. Luis Carvajal-Huerta [15], a deletion in desmoplakin (DSP) gene was identified by Norgett et al., as the causative mutation [6]. Other recessive desmoplakin mutations (R1267X and p.Gln1301X) or compound desmoplakin heterozygosity (Q1446X plus Q673X) largely truncating desmoplakin molecule at its C-terminal, have been reported.
The ARVC/D of Naxos disease shows 100% penetrance [20]. Mutation in genes encoding the adhesion molecules results in weakening and disruption of desmosomes and adherens junctions of myocardium and epidermis predominantly [10]. As a result, cell death and apoptosis occurs compromising the integrity of the tissues in stress. Subsequent fibrofatty replacement of myocardium leads to dilation and aneurysm formation in ventricles leading to progressive cardiac failure. Disintegrated gap junctions at cellular level, and fibrofatty nidi within the myocardium at tissue level cause disturbances in electrical integrity, arrhythmia and sudden death. Stress induced tissue loss at pressure points in limbs explain the occurrence of palmoplantar keratoderma.
Recently, variants of Naxos disease-Carvajal syndrome with dental and/or other anomalies have been reported [21-25]. A form of ARVC/D characterized by phenotypic features overlapping Naxos/Carvajal syndrome, with a dominant mode of inheritance has also been described [26]. Besides these, novel mutations have been reported in association with Naxos disease and Carvajal syndrome [17-27].
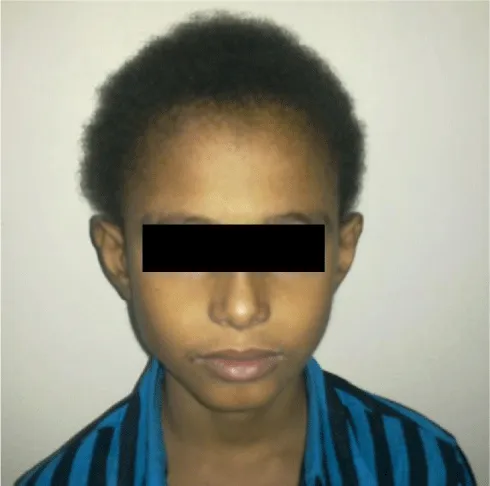
Pathology: Woolly hair is one of the telltale signs of Naxos disease and Carvajal syndrome. This is the first of the triad of phenotypes to appear, and is present since birth. The peculiar hair is readily evident in the scalp, and is extremely curly, thin, grows slowly, often fragile, and is difficult to comb. However, woolly hair is not specific to Naxos disease and Carvajal syndrome; woolly hair can be autosomal dominant (hereditary form), autosomal recessive (familial wooly hair), and localized nonhereditary woolly hair in the form of woolly hair nevus [28]. (Figure 1).
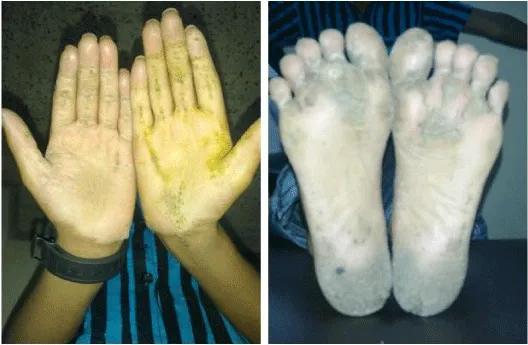
Like the woolly hair, palmoplantar keratoderma is a characteristic feature of Naxos disease and Carvajal syndrome. Palmoplantar keratoderma is characterized by thickening of the skin on the palms and soles. This feature is not present at birth, rather develops during the first year of life when the infant starts using the hands and feet [19]. Palmoplantar keratoderma of Naxos disease and Carvajal syndrome is of striate type, however, is not specific to them, and can be seen in other acquired and inherited conditions (Figure 2).
Cardiomyopathy is the most clinically important feature of Naxos disease and Carvajal syndrome. The cardiomyopathy of both the conditions constitutes the relatively rare, recessively-transmitted variant of ARVC/D, which can affect either ventricle. In Naxos disease, the RV is affected, and usually presents by adolescence with arrhythmia manifested as syncope and/or ventricular tachycardia (VT) of left bundle branch block (LBBB) morphology. In contrast, in case of Carvajal syndrome, the LV is predominantly affected, the presentation is in earlier ages, may be in childhood, and the usual presentation is heart failure rather than arrhythmia. Presence of myocardial non-compaction [29] and absence of significant myocardial fibro-fatty replacement [30], also differentiate cardiomyopathy in Carvajal syndrome from that in Naxos disease.
Diagnosis
The diagnosis of Naxos disease and Carvajal syndrome is often straight forward in presence of telltale features usually in childhood or adolescence. Woolly hair and palmoplantar keratoderma are recognized clinically. No single diagnostic test exists for ARVC/D. The diagnosis is made using a combination of clinical, electrocardiographic and radiological features, as defined by the 2010 Task Force Criteria (TFC) [31].
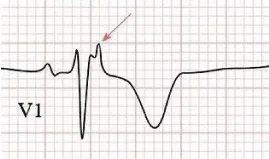
Electrocardiograms (ECGs), echocardiography and cardiac magnetic resonance (CMR) are the investigations commonly done for diagnosis and evaluation of ARVC/D. ECG findings usually are the first clinical abnormalities recognized in patients suspected of ARVC/D. The classic ECG findings in ARVC/D are inverted T waves in the right precordial leads (V1-V3) with an epsilon wave just after the QRS in lead V1 [32]. Complete and incomplete right bundle branch block (RBBB) may also be observed [33,34]. The epsilon waves are reproducible small deflections seen just beyond the QRS complex in lead V1 or V2. (Figure 3). Signal-averaged ECG (SAECG) may show late potentials. An abnormal SAECG is supportive of the diagnosis of ARVC/D but a negative test does not exclude this diagnosis. Holter ECG assists with the diagnosis when there are frequent ventricular extra-systoles, (>1,000 per 24 h) usually of LBBB morphology. Ventricular tachycardia (VT) occurring in ARVC/D patients originate in the RV, is of LBBB morphology and has either an inferior or superior QRS axis [35]. The major differential diagnosis of VT of LBBB morphology with inferior QRS axis (negative QRS in lead aVL) is relatively benign right ventricular outflow tract (RVOT) tachycardia. Patients with RVOT tachycardia do not usually have the ECG abnormalities associated with ARVC/D and the SAECG is generally normal.
Echocardiography and CMR detect structural and functional abnormalities of RV or LV or both. A transthoracic echocardiography (TTE) examination is an ideal screening tool to assess ventricular size and function in patients with possible ARVC/D or LV cardiomyopathy. In case of ARVC/D, the TTE features include RV dilation, aneurysm formation, and global or regional hypokinesia mainly in the sub-tricuspid region, RVOT and RV apex [36]. Echocardiography is sensitive for ARVC/D, but it is not specific, because it does not allow for an assessment of the adipose substitution of the myocardium, which is a hallmark of ARVC/D. Echocardiography is less sensitive than CMR, because it may fail to depict focal RV wall thickening, and it does not reliably help in distinguishing fat from muscle. Three-dimensional (3D) echocardiography, contrast echocardiography, tissue-Doppler imaging, strain-rate imaging may play role in future. In case of Carvajal syndrome, echocardiographic features include dilated cardiomyopathy, may be having features of non-compaction predominantly affecting the LV.
CMR is the non-invasive tool of choice; it enables good assessment of cardiac morphology and function while also allowing flow dynamic studies and tissue characterization. CMR findings associated with ARVC/D include RV wall thinning, RVOT enlargement, trabecular disarray, fibrofatty replacement, ventricular dilation, and global or regional systolic dysfunction [37-39]. These abnormalities typically occur in predilection sites including the RV base and LV lateral wall. Like RV evaluation, CMR is useful in evaluation of LV cardiomyopathy in Carvajal syndrome as well. The CMR picture of Carvajal syndrome often mimics that of LV non- compaction [30].
Cardiac computed tomography (CT) scanning is not included in the 2010 TFC for ARVC/D. However, a possible use of CT lies in the evaluation of claustrophobic patients, assessment of subjects with frequent ventricular extra-systoles leading to severe arrhythmia artifacts on CMR, serial evaluation of automated implantable cardioverter-defibrillator (AICD) carriers, and before ablation procedures [40,41].
Electrophysiological studies are not included in the 2010 TFC, but may be important for differential diagnosis from RVOT tachycardia. Ventriculography, endomyocardial biopsy and genetic testing may be indicated in selected cases.
Treatment
Like ARVC/D, the most important goals of clinical management of Naxos disease and Carvajal syndrome patients comprise: reduction of mortality, either by arrhythmic sudden death or death due to heart failure; prevention of disease progression leading to RV, LV or biventricular dysfunction and heart failure; attenuation of symptoms and improvement of quality of life by decreasing or suppressing palpitations, VT recurrences or AICD discharges; and reducing heart failure symptoms and increasing exercise capacity. Life-style modifications, pharmacologic treatment, catheter ablation, AICD implantation, and exceptionally heart transplantation are the management options. In case of Naxos disease, syncope, LV involvement and the appearance of symptoms and/or structural progression before the age of 35 years were risk factors for sudden death [20]. Sustained or non-sustained VT and decreased LV function have recently been identified as risk factors for subsequent arrhythmic death in patients with ARVC/D [42]. Endocardial voltage mapping (EVM) has been found to be useful in stratifying the arrhythmia risk in ARVC/D patients. The extent of negative T-waves across the 12-lead ECG was found to correlate with the amount of RV-electroanatomic scar (RV-EAS) and predict EAS-related arrhythmic risk [43]. Also, the extent of bipolar RV endocardial low-voltage area detected by EVM was shown to be a powerful predictor of arrhythmic outcome in ARVC/D, independently of history and RV dilatation/dysfunction; a normal bipolar EVM characterized a low-risk subgroup of ARVC/D patients [44]. Anti-arrhythmics like sotalol and amiodarone are used. However, AICD implantation is the most effective strategy for interruption of potentially lethal ventricular tachy-arrhythmias in cardiocutaneous syndrome. For ARVC/D, there is general agreement that patients with a history of cardiac arrest or hemodynamically unstable VT are at high risk of sudden cardiac death and needs an AICD, however, indications for primary prevention remain a matter of debate [45]. For Naxos disease and Carvajal syndrome, AICD is probably indicated in those who develop symptoms and/or structural progression before the age of 35 years [17,20,46,47]. Anti-arrhythmic drugs are used as an adjunct after AICD implantation. The wearable cardioverter-defibrillators (WCDs) may have a role as a bridging therapy before the scheduled AICD implantation or heart transplantation in Naxos disease and Carvajal syndrome [48,49].
Diuretics and angiotensin converting enzyme inhibitors are indicated in heart failure, anti-thrombotics may prevent thrombus formation in high-risk patients. Heart transplantation remains the only option for the end-stage cardiomyopathy patients [12]. Ventricular assist device (VAD) has been used before cardiac transplantation in a patient with Carvajal syndrome [50]. Emollients, topical keratolytics, tazarotene 0.05%, and tretinoin 0.1% can be tried for palmoplantar keratoderma [51].
Prevention
So far, 13 disease genes have been identified, responsible for around 60% of all ARVC/D cases [52]. Most of mutations in dominant forms have been identified in desmosomal genes including DSP, plakophilin-2 (PKP2), desmoglein-2 (DSG2), desmocollin-2 (DSC2) and JUP [53]. Being genetic diseases, ARVC/D is at present incurable. So, early detection and prevention may be attractive options. Clinical genetic testing is already available worldwide for the common ARVC/D-associated genes, e.g., plakophilin-2 (PKP2), desmoplakin (DSP), desmoglein-2 (DSG2), desmocollin-2 (DSC2), plakoglobin (JUP), transmembrane protein 43 (TMEM43), ryanodine receptor 2 (RYR2) [54]. However, genotyping success rate in ARVC/D varies according to cohort location and ethnicity, sequencing techniques, selection criteria and the stringency of the standards by which mutations are considered causal [53]. Thus, genetic testing and its interpretation should be performed in dedicated ARVC/D cardio-genetic centers, with pre- and post-counseling facilities. A negative genetic test does not exclude a genetic predisposition.
In an attempt to control Naxos disease, systematic genetic screening of the populations at risk has been carried out to identify the heterozygous carriers of the plakoglobin gene mutation [1]. Several registries are dealing with ARVC/D patients around the globe. A multidisciplinary collaborative European study has been designed with the aim to investigate the clinical, pathological and genetic features of ARVC/D [55]. One of the missions is the study of Naxos disease (participating unit: Yannis Protonotarios Medical Center, Naxos, Greece). Simultaneously, another major registry in North America worked in collaboration with European expertise [56]. The Johns Hopkins Arrhythmogenic Right Ventricular Dysplasia (Cardiomyopathy) Program of USA [54], the Australian Genetic Heart Disease Registry [57], the Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) Program at the University Hospital Zurich [58] and the Canadian Arrhythmogenic Right Ventricular Cardiomyopathy Registry [59], are also active.
Conclusion
The liaison between skin and heart may be dangerous. The clinicians should have appropriate preparedness to recognize the rare but often lethal cardiocutaneous syndromes including Naxos disease and Carvajal syndrome. Any child presenting with woolly hair at birth should be evaluated and followed up regularly. The mainstay of treatment is to prevent sudden cardiac death. AICD should be offered to the high risk cases. Early diagnosis, proper risk stratification, timely treatment, and regular follow up can lessen the morbidity and mortality of the patients.
- Protonotarios N, Tsatsopoulou A (2006) Naxos disease: cardiocutaneous syndrome due to cell adhesion defect. Orphanet J Rare Dis 1: 4. Link: https://goo.gl/6OeiWu
- Simpson MA, Mansour S, Ahnood D, Kalidas K, Patton MA, et al. (2009) Homozygous mutation of desmocollin-2 in arrhythmogenic right ventricular cardiomyopathy with mild palmoplantar keratoderma and woolly hair. Cardiology 113: 28-34. Link: https://goo.gl/rzd9bx
- Awad MM, Dalal D, Tichnell C, James C, Tucker A, et al. (2006) Recessive arrhythmogenic right ventricular dysplasia due to novel cryptic splice mutation in PKP2. Hum Mutat 27: 1157. Link: https://goo.gl/V9HlSa
- Protonotarios N, Tsatsopoulou A. (2004) Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Pathol 13: 185-194. Link: https://goo.gl/36Xacb
- McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, et al. (2000) Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 355: 2119-2124. Link: https://goo.gl/8Foj2X
- Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, et al. (2000) Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet 9: 2761-2766. Link: https://goo.gl/UXgJ2h
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, et al. (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 93(5):841-842. Link: https://goo.gl/i3nY6i
- Protonotarios N, Tsatsopoulou A, Patsourakos P, Alexopoulos D, Gezerlis P, et al. (1986) Cardiac abnormalities in familial palmoplantar keratosis. Br Heart J 56: 321-326. Link: https://goo.gl/lyHkwN
- Islam AM, Rahman MT, Chowdhury AH. (2016) Cardiocutaneous syndrome (Naxos disease) in a Bangladeshi boy. Cardiovasc Diagn Ther 6: 462-465. Link: https://goo.gl/vltUKx
- Saravanan RR, Amuthan V, Janarthanan RA, Balasubramanian S, Mohamed SN. (2012) A case of arrhythmogenic right ventricular cardiomyopathy-Naxos disease. Indian Heart J 64: 84-87. Link: https://goo.gl/lUAhDa
- Bukhari I, Juma'a N (2004) Naxos disease in Saudi Arabia. J Eur Acad Dermatol Venereol 18: 614-616. Link: https://goo.gl/zqRSSe
- Narin N, Akcakus M, Gunes T, Celiker A, Baykan A, et al. (2003) Arrhythmogenic right ventricular cardiomyopathy (Naxos disease): report of a Turkish boy. Pacing Clin Electrophysiol 26: 2326-2329. Link: https://goo.gl/TEys93
- Djabali K, Martinez-Mir A, Horev L, Christiano AM, Zlotogorski A (2002) Evidence for extensive locus heterogeneity in Naxos disease. J Invest Dermatol 118: 557-560. Link: https://goo.gl/h77BO9
- Madhu KJ, Vijyalakshmi IB, Narsimhan C, Manjunath CN. (2016) Carvajal Syndrome: A Rare Variant of Naxos Disease. Cardiovas Pathol 1: 103. Link: https://goo.gl/GYogKR
- Carvajal-Huerta L (1998) Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol 39: E418-421. Link: https://goo.gl/VJElpW
- Rao BH, Reddy IS, Chandra KS (1996) Familial occurrence of a rare combination of dilated cardiomyopathy with palmoplantar keratoderma and woolly hair. Indian Heart J 48: 161-162. Link: https://goo.gl/BVkIqz
- Molho-Pessach V, Sheffer S, Siam R, Tams S, Siam I, et al. (2015) Two novel homozygous desmoplakin mutations in Carvajal syndrome. Pediatr Dermatol 32: 641-646. Link: https://goo.gl/aHsGAI
- Kilic T, Babaoglu K, Aygün F, Vural A, Ural D, et al. (2007) Biventricular involvement in a Turkish boy with palmoplantar hyperkeratosis and curly hair, an unusual presentation of Naxos-Carvajal syndrome. Int J Cardiol 115: e122-125. Link: https://goo.gl/KYWfys
- Coonar AS, Protonotarios N, Tsatsopoulou A, Needham EW, Houlston RS, et al. (1998) Gene for arrhythmogenic right ventricular cardiomyopathy with diffuse nonepidermolytic palmoplantar keratoderma and woolly hair (Naxos disease) maps to 17q21. Circulation 97: 2049- 2058. Link: https://goo.gl/rKWnhm
- Protonotarios N, Tsatsopoulou A, Anastasakis A, Sevdalis E, McKoy G, et al. (2001) Genotype-phenotype assessment in autosomal recessive arrhythmogenic right ventricular cardiomyopathy (Naxos disease) caused by a deletion in plakoglobin. J Am Coll Cardiol 38: 1477-1484. Link: https://goo.gl/VV0Z5T
- Stöllberger C, Vujic I, Wollmann E, Freudenthaler J, Finsterer J. (2016) Carvajal syndrome with oligodontia, hypoacusis, recurrent infections, and noncompaction. Int J Cardiol 203: 825-827. Link: https://goo.gl/h3hJRo
- Boulé S, Fressart V, Laux D, Mallet A, Simon F, et al. (2012) Expanding the phenotype associated with a desmoplakin dominant mutation: Carvajal/Naxos syndrome associated with leukonychia and oligodontia. Int J Cardiol 161: 50-52. Link: https://goo.gl/qot2B1
- Nehme N, El Malti R, Roux-Buisson N, Caignault JR, Bouvagnet P. (2012) Evidence for genetic heterogeneity in Carvajal syndrome. Cell Tissue Res. 348(2): 261-264. Link: https://goo.gl/AYynNW
- Barber S1, Day P, Judge M, Toole EO, Fayle S. (2012) Variant Carvajal syndrome with additional dental anomalies. Int J Paediatr Dent 22: 390-396. Link: https://goo.gl/u9wh3y
- Chalabreysse L, Senni F, Bruyère P, Aime B, Ollagnier C, et al. (2011) A new hypo/oligodontia syndrome: Carvajal/Naxos syndrome secondary to desmoplakin-dominant mutations. J Dent Res 90: 58-64. Link: https://goo.gl/1l77bD
- Tomberli B, Fornaro A, Bardi S, Torricelli F, Benelli M, et al. (2013) A novel desmoplakin dominant mutation responsible for Carvajal/Naxos syndrome identified by exome sequencing. Eur Heart J 34: P2959; Link: https://goo.gl/lQJe1r
- Bitar F, Najjar T, Hayashi R, Nemer G, Shigehara Y, et al. (2016) A novel heterozygous mutation in desmoplakin gene in a Lebanese patient with Carvajal syndrome and tooth agenesis. J Eur Acad Dermatol Venereol 30: e217-e219. Link: https://goo.gl/Li9vha
- Olsen EA (2011) Hair Disorders. In Text Book of Pediatric Dermatology. 3rd edition. Edited by Irvine AD, Hoeger PH, Yan AC, Oxford: Willey-Blackwell 148.1. Link: https://goo.gl/XYfOss
- Kaplan SR, Gard JJ, Carvajal-Huerta L, Ruiz-Cabezas JC, Thiene G, et al. (2004) Structural and molecular pathology of the heart in Carvajal syndrome. Cardiovasc Pathol 13: 26-32. Link: https://goo.gl/ObiVG7
- Prompona M, Kozlik-Feldmann R, Mueller-Hoecker J, Reiser M, Huber A. (2007) Images in cardiovascular medicine. Magnetic resonance imaging characteristics in Carvajal syndrome (variant of Naxos disease). Circulation 116: e524-530. Link: https://goo.gl/Fs8SIu
- Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, et al. (2010) Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J 31: 806-14. Link: https://goo.gl/vXcWVs
- Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, et al. (2009) AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 53: 976-981. Link: https://goo.gl/vbA7Iz
- Jain R, Dalal D, Daly A, Tichnell C, James C, et al. (2009) Electrocardiographic features of arrhythmogenic right ventricular dysplasia. Circulation 120: 477-487. Link: https://goo.gl/ryxIeO
- Nasir K, Bomma C, Tandri H, Roguin A, Dalal D, et al. (2004) Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: a need to broaden diagnostic criteria. Circulation 110: 1527-1534. Link: https://goo.gl/cTMshq
- Ainsworth CD, Skanes AC, Klein GJ, Gula LJ, Yee R, et al. (2006) Differentiating arrhythmogenic right ventricular cardiomyopathy from right ventricular outflow tract ventricular tachycardia using multilead QRS duration and axis. Heart Rhythm 3: 416-423. Link: https://goo.gl/nxKz4d
- Baran A, Nanda NC, Falkoff M, Barold SS, Gallagher JJ (1982) Two-dimensional echocardiographic detection of arrhythmogenic right ventricular dysplasia. Am Heart J 103: 1066-1067. Link: https://goo.gl/0XBRvu
- te Riele AS, Tandri H, Bluemke DA (2014) Arrhythmogenic right ventricular cardiomyopathy: cardiovascular magnetic resonance update. J Cardiovasc Magn Reson 16: 50. Link: https://goo.gl/uKcgEY
- Jain A, Tandri H, Calkins H, Bluemke DA (2008) Role of cardiovascular magnetic resonance imaging in arrhythmogenic right ventricular dysplasia. J Cardiovasc Magn Reson 10: 32. Link: https://goo.gl/UcWmaQ
- Tandri H, Bomma C, Calkins H, Bluemke DA (2004) Magnetic resonance and computed tomography imaging of arrhythmogenic right ventricular dysplasia. J Magn Reson Imaging 19: 848-858. Link: https://goo.gl/t7P3JL
- Te Riele AS, Tandri H, Sanborn DM, Bluemke DA (2015) Noninvasive Multimodality Imaging in ARVD/C. JACC Cardiovasc Imaging. 8(5): 597-611. Link: https://goo.gl/9D8cvc
- Komatsu Y, Jadidi A, Sacher F, Denis A, Daly M, Derval N, et al. (2014) Relationship between MDCT-imaged myocardial fat and ventricular tachycardia substrate in arrhythmogenic right ventricular cardiomyopathy. J Am Heart Assoc 3: pii: e000935. Link: https://goo.gl/Xlulti
- Brun F, Groeneweg JA, Gear K, Sinagra G, van der Heijden J, et al. (2016) Risk stratification in Arrhythmic right ventricular cardiomyopathy without implantable cardioverter-defibrillators. JACC Clin Electrophysiol 2: 558-564. Link: https://goo.gl/IyUAWD
- Zorzi A, Migliore F, Elmaghawry M, Silvano M, Marra MP, et al. (2013) Electrocardiographic predictors of electroanatomic scar size in arrhythmogenic right ventricular cardiomyopathy: implications for arrhythmic risk stratification. J Cardiovasc Electrophysiol 24: 1321-1327. Link: https://goo.gl/jBRxv3
- Migliore F, Zorzi A, Silvano M, Bevilacqua M, Leoni L, et al. (2013) Prognostic value of endocardial voltage mapping in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Arrhythm Electrophysiol 6: 167-176. Link: https://goo.gl/jWhrNQ
- Zorzi A, Rigato I, Bauce B, Pilichou K, Basso C, et al. (2016) Arrhythmogenic right ventricular cardiomyopathy: Risk stratification and indications for defibrillator therapy. Curr Cardiol Rep 18: 57. Link: https://goo.gl/7T0Vwf
- Gatzoulis K, Protonotarios N, Anastasakis A, Tsatsopoulou A, Vlasseros J, et al. (2000) Implantable defibrillator therapy in Naxos disease. Pacing Clin Electrophysiol 23: 1176-1178. Link: https://goo.gl/nXWGG3
- Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, et al. (2003) Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 108: 3084-3091. Link: https://goo.gl/H5jaP4
- Piccini JP Sr, Allen LA, Kudenchuk PJ, Page RL, Patel MR, et al. (2016) Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death: A Science Advisory From the American Heart Association. Circulation 133: 1715-1727. Link: https://goo.gl/D2OzOl
- Wäßnig NK, Günther M, Quick S, Pfluecke C, Rottstädt F, et al. (2016) Experience with the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circulation 134: 635-643. Link: https://goo.gl/Rlqhaz
- Bottio T, Bejko J, Tarzia V, Gerosa G. (2014) HeartWare LVAD implantation in a patient with a rare ARVD: Carvajal syndrome. Int J Artif Organs 37: 563-566. Link: https://goo.gl/XnjlXc
- Srinivas SM, Kumar P, Basavaraja GV (2016) Carvajal syndrome. Int J Trichology 8: 53-55. Link: https://goo.gl/LQKwfY
- Poloni G, De Bortoli M, Calore M, Rampazzo A, Lorenzon A (2016) Arrhythmogenic right-ventricular cardiomyopathy: molecular genetics into clinical practice in the era of next generation sequencing. J Cardiovasc Med (Hagerstown) 17: 399-407. Link: https://goo.gl/8JcIqu
- Pilichou K, Thiene G, Bauce B, Rigato I, Lazzarini E, et al. (2016) Arrhythmogenic cardiomyopathy. Orphanet J Rare Dis 11: 33. Link: https://goo.gl/XBvOUh
- The Johns Hopkins Arrhythmogenic Right Ventricular Dysplasia (Cardiomyopathy) Program. Heart & Vascular Institute. John Hopkins Medicine Link: https://goo.gl/2b3YEC
- Basso C, Wichter T, Danieli GA, Corrado D, Czarnowska E, et al. (2004) Arrhythmogenic right ventricular cardiomyopathy: clinical registry and database, evaluation of therapies, pathology registry, DNA banking. Eur Heart J 25: 531-534. Link: https://goo.gl/3NsWNT
- Marcus F, Towbin JA, Zareba W, Moss A, Calkins H, et al. (2003) Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C): a multidisciplinary study: design and protocol. Circulation 107: 2975-2978. Link: https://goo.gl/5H41LB
- The Australian Genetic Heart Disease Registry. Link: https://goo.gl/12Dh8d
- Zurich ARVC Program. Link: https://goo.gl/7qKsxS
- Krahn AD, Healey JS, Gerull B, Angaran P, Chakrabarti S, et al. (2016) The Canadian Arrhythmogenic Right Ventricular Cardiomyopathy Registry: Rationale, design, and preliminary recruitment. Can J Cardiol 32: 1396-1401. Link: https://goo.gl/kxTCjl

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
