Global Journal of Infectious Diseases and Clinical Research
Organic and inorganic light-emitting diodes for photodynamic therapy of cutaneous Leishmaniasis
Saydulla Persheyev1, Ifor Samuel1* and Terry Smith2*
2Schools of Biology & Chemistry, BSRC, University of St Andrews, St Andrews, Fife KY16 9ST, UK
Terry Smith, Schools of Biology & Chemistry, BSRC, University of St Andrews, St Andrews, Fife KY16 9ST, UK, Tel: 01334-463412; E-mail: [email protected]
Cite this as
Persheyev S, Samuel I, Smith T (2023) Organic and inorganic light-emitting diodes for photodynamic therapy of cutaneous Leishmaniasis. Glob J Infect Dis Clin Res 9(1): 025-030. DOI: 10.17352/2455-5363.000058Copyright
© 2023 Persheyev S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.For effectively fighting worldwide infectious diseases such as cutaneous Leishmaniasis, novel approaches are required. Photodynamic Therapy (PDT) is one such possibility. PDT involves applying a light-sensitive chemical (photosensitizer), which should be highly efficient, non-toxic, and work at longer light wavelengths. This photosensitizer needs to be activated by a light source that provides uniform emission over a large area, high intensity, easy to fabricate, compact, and low cost. In this work, we designed and built light sources based upon commercially available Organic Light Emitting Diode (OLED) and LED parts to experimentally validate the combination with methylene blue photosensitizer to kill Leishmania major and Crithidia fasciculata cells in vitro. Our results showed that suitable-sized OLEDs, as compact and uniform light sources, are very good candidates for photodynamic therapy and can be used to efficiently kill such kinetoplastids in vitro. Therefore, it has real potential to be used in wearable devices for ambulatory treatment of patients.
Introduction
Leishmaniasis is an important parasitic disease that affects around two million people every year, mainly in developing countries [1-5]. Effective and affordable treatment is needed, and Photodynamic Therapy (PDT) is a promising approach. In photodynamic therapy, a light source in combination with a Photosensitizer (PS) is used to generate reactive oxygen species [6-10] which destroy parasites. Methylene blue is efficient and a well-known PS, licensed by the FDA as a topical agent, and has maximum absorption in the red-light spectra.
There are a small number of chemotherapeutic treatments for visceral and cutaneous Leishmaniasis, with miltefosine being the most recent addition that can be used to treat both. Its mechanism of action is not well understood [11-13] and given it has an increasing problem with resistance in the field [14] and common side effects such as vomiting, diarrhea, dizziness, skin reactions and is not recommended to people with kidney, liver diseases, or expectant mothers, there is an urgent need for alternative therapeutic approaches [1-3,15] such as photodynamic therapy.
Since the late 1970s, PDT has been under development and has been intensively used in oncology including dermatology, surgery, urology, and many more [16-19]. PDT experimental data on the treatment of Leishmanial diseases is still limited and cannot so far be recommended in routine clinical practice, more laboratory-based research is required to validate the treatment [15,20]. In the case of cutaneous Leishmaniasis, when parasites affect mainly the skin surface and dermis [21], the possibility of effective treatment of the infected area with PDT is more viable [19,22-24]. Crithidia fasciculata is a free-swimming kinetoplastid and is not infective to vertebrates [25]. It is an example of a non-human infective trypanosomatid but is closely related to several human parasites including Leishmania spp, which cause Leishmaniasis, and for that reason is often used to test new therapeutic strategies against parasitic infections [26].
The aim of this paper is to explore the potential of OLED and LED-based light sources for PDT to kill promastigotes of Leishmania major and Crithidia fasciculata cells, Light sources for PDT are usually large and bulky. Instead, we have designed and realized small, portable, efficient light sources and used them for in-vitro studies in 96 well plates with the photosensitizer (MB).
Experimental
Light sources
The requirements for light sources used for PDT could be described as follows - high intensity, high uniformity, and spectral conformity to the absorption spectra of the photosensitizer. High intensity of the light sources is usually required to reduce exposure times, it is particularly important when sources are used for light treatment of humans and on animal tests when exposure times cannot exceed reasonable times enabling animals to receive a treatment. In the meantime, intensities around 100 mW/cm2 and higher are not advised due to the burning sensation, erythema [17] on patients, and PS bleaching effects [27]. High uniformity of the source is required for the homogeneous distribution of photon energy to photosensitizer used for higher quantitative estimation delivered optical power [19,22].
Table 1 shows some of the previously published data on light sources used for PDT on various Leishmania species in the last 20 years. Laser-based light sources have high output power parameters but are strictly limited.
The cost of laser systems also limits the applications for these types of devices and is a particular concern as Leishmaniasis is prevalent in developing countries. Broadband light sources are bulky and expensive, generally for laboratory use only, and are often not efficient. The most commonly used devices are inorganic LEDs, due to their high-power output, high efficiency, and low cost compared to other easily available devices, but still large machines.
Organic light-emitting diode light sources (OLED) attracting more attention for using biomedical applications such as antimicrobial PDT due to high uniformity, large area, flexibility, and increased lifetime [32].
We have designed and built our light emitting devices for PDT using OLED and LED (Lighting panel, Red, KANEKA Corp., and Red LED, CREE) based upon devices used for in vitro experiments that utilize common and useful 96-well plates for culturing and testing of cells including single cells such as Leishmania major and Crithidia fasciculata. We have analyzed different lighting approaches and came to the conclusion that underneath or bottom-up exposure of cells in the well plates is preferable because it allows to delivery of higher illumination intensities to the media containing the PS and cells, due to the shorter distance from the source (Supplementary Data Figure 1), thus avoiding use of high currents in our devices. High currents are undesirable because they shorten device lifetime and cause device heating, initiate aging and decrease the OLED lifetime of organic devices, and can affect the ambient temperature inside well plates, both of which we wish to minimize.
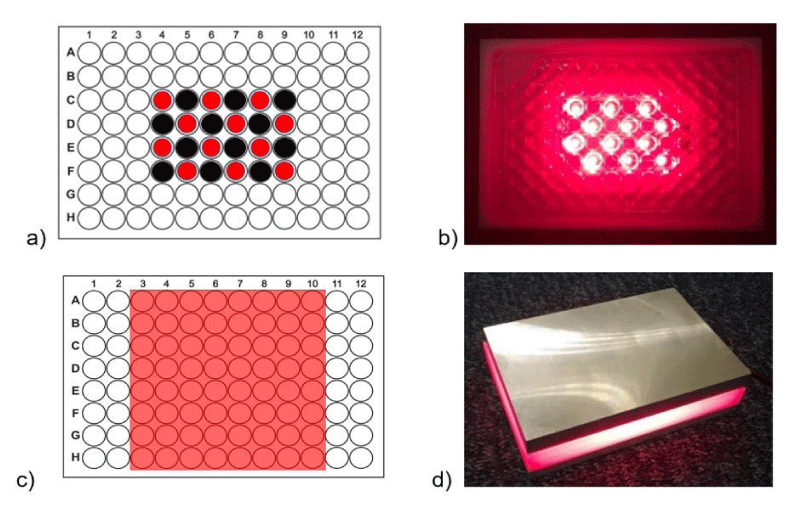
For easy and practical illumination of the live cells in vitro we have built the light sources based upon high performance, large area, high uniformity OLEDs, and using off-the-shelf high power LEDs. The wavelength of the sources was selected to match the spectral adsorption property of the chosen PS, i.e. methylene blue (662 nm) used in our experiments (Supplementary Data Figure 2). As can be seen from the figure, the methylene blue maximum absorbance overlaps with a local maximum of both the LED and OLED sources. This wavelength overlap of the methylene blue provides very good penetration through the human skin as well. The experimental LED setup accommodates 96-well plates and illuminates simultaneously 12 wells (Figure 1a,b) uniformly from underneath, with variable power ranging from 12.5, 25 up to 50 mW/cm2.
The optimal distance from LED to the 96-well plate was chosen to be 4 mm, it permits emission intensity tolerance to be around ±5% when the incident angle does not exceed 40 degrees (Supplementary Data Figure 3). It is a very acceptable deviation of the optical power delivered to the cells in such experiments.
For large-area OLED devices, we used “Kaneka Corp.” (part number 159002443A32, Red) high stability and uniform emitting devices with an emitting area of 80 mm x 80 mm, allowing simultaneous illumination of 64 out of 96 wells in the plate (Figure 1c,d) with fixed output intensity of around 2 mW/cm2. During the exposure process, the temperature was monitored and did not go higher than 28 oC.
Other advantages include being an area light source instead of a point source for LEDs, being thin, and lightweight, and having the potential to be flexible or conformable to wear directly on the skin area being treated.
Photosensitizer
In photodynamic therapy, photosensitizer absorbs the energy of the photon and through the chain of energy, transfers cause the production of cytotoxic radicals such as singlet oxygen and oxygen reactive species. The more radicals created in the process of light absorption, the more effective the PS. Nontoxicity to cells, and biocompatibility of the PS when it is not illuminated with light are also of great importance for developing new therapies against leishmaniosis. Methylene blue (0-200 mm, pre-incubated for 30 minutes) was chosen as the best candidate in our case for the effective killing of L. major and C. fasciculata in vitro with a maximum of absorption around 662 nm wavelength.
Cells and culture
Crithidia fasciculata clone HS6 was cultured at 27 oC with gentle agitation in a liquid medium defined below in non-vented flasks. Cells were passaged to sustain mid-log phase growth by transferring cells into fresh media before the density reached ~5 x 107 cells/mL [26]. Promastigotes from Leishmania major Freidlin strain were grown at 28 oC in M199 medium pH 7.4, supplemented with 40 mM HEPES pH 7.4, 100 μM adenosine, 5 μg/mL haemin, and 10% heat-inactivated fetal bovine serum. Cells were passaged every 2-3 days to maintain mid-log growth.
Cell viability
Alamar Blue cell viability reagent was used for quantitative fluorescence live/dead assays to measure the toxicity of methylene blue photosensitizer as part of PDT, against both L. major and C. fasciculata [26].
The cell viability was assessed with Alamar blue 24 h after control or PDT treatment. All assays were conducted in replicates of three or four.
The Alamar Blue assay allows a simple, reproducible method for PDT experiments [33]. When added to cells, Alamar Blue is metabolized by the reducing environment of viable cells turns red in color, and becomes highly fluorescent. Fluorescence measurements were performed with the Spectra Max Gemini XPS Microplate reader using an excitation between 530–560 nm and an emission at 590 nm.
Results
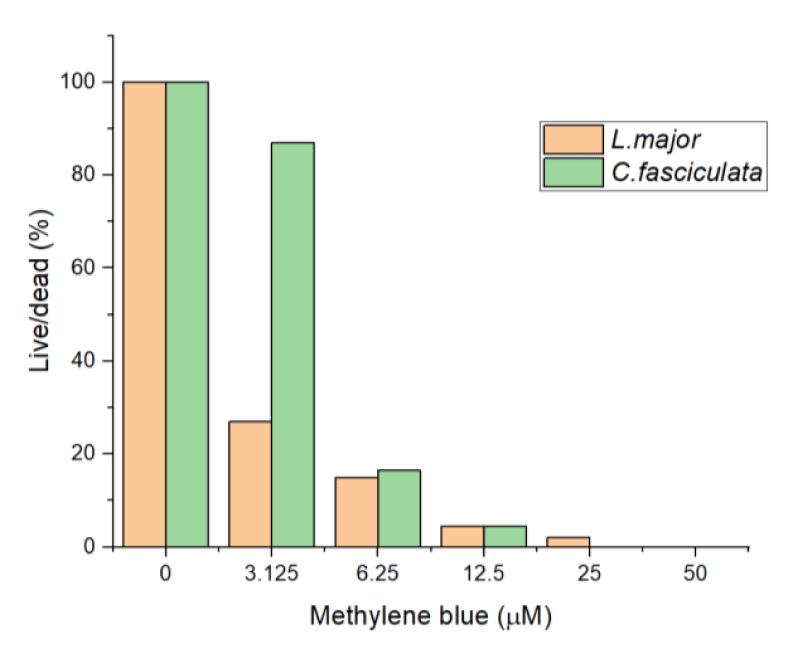
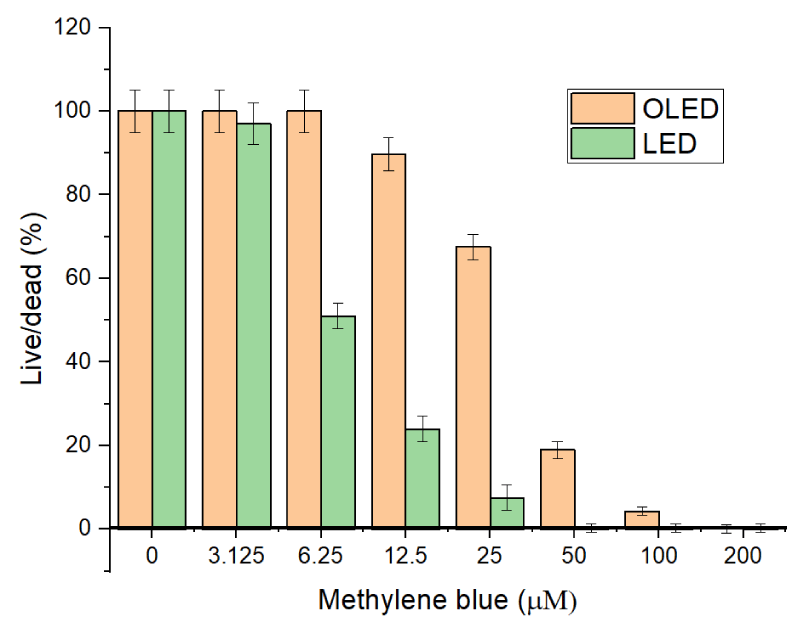
Initially, in our experiments, the L. major and C. fasciculata were illuminated at the highest intensity of LED light source 50 mW/cm2 and the exposure time was 30 minutes with varying concentrations of the MB in the solution; 3.15, 6.25, 12.5, 25 and 50 µM. Two control groups were done in parallel: the first one was cells that did not have any MB introduced, just exposed to light, and the second with no light, only with MB. Cells in control groups were not affected after 30 minutes. As we can see from our results (Figure 2), C. fasciculata demonstrated a more resistive nature to PDT at concentrations of MB up to 25 µM.
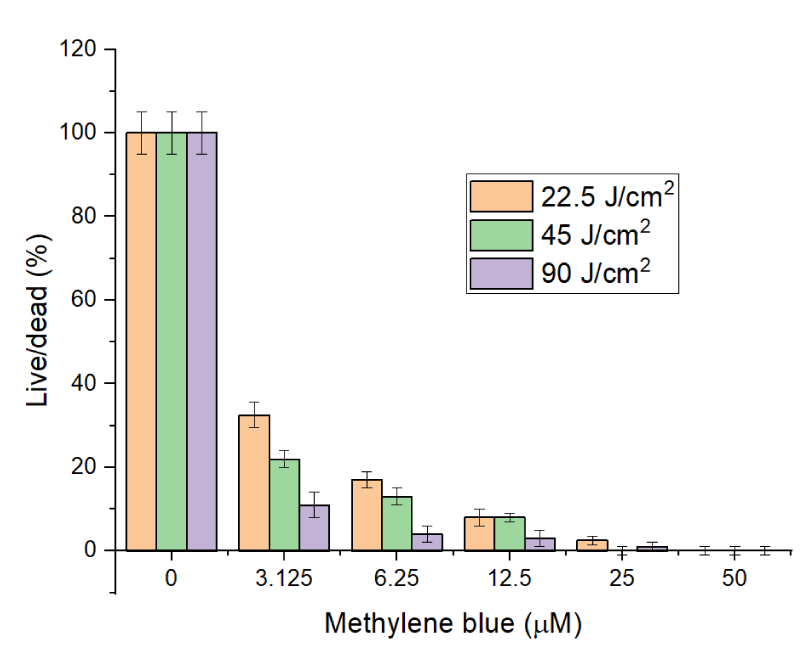
Next, the exposure of C. fasciculata cells to different fluences ranging from 22.5 to 45 and 90 J/cm2 (accordingly exposure times 7.5, 15, and 30 minutes) while the light intensity was kept constant at 50 mW/cm2 (Figure 3) was investigated.
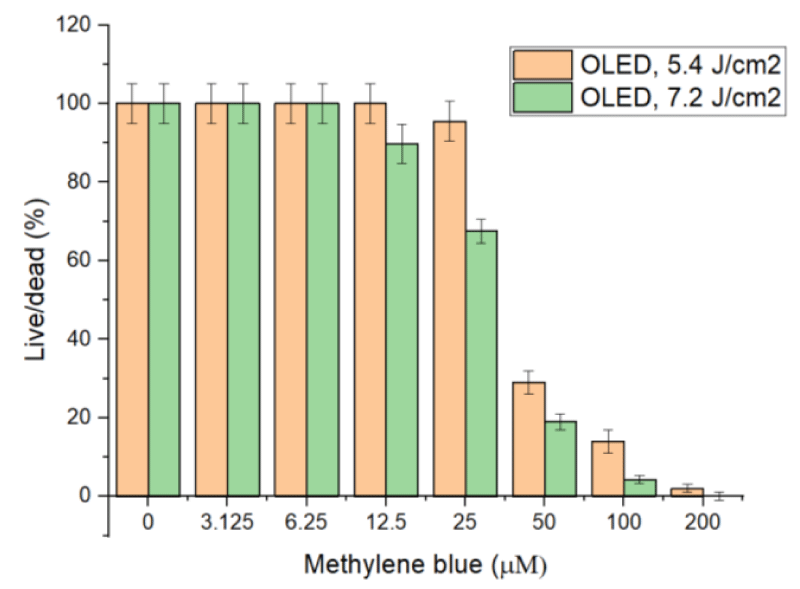
Figure 3 shows how the higher doses increase the killing rate of C. fasciculata, such that at concentrations 50 µM and above, PDT with MB generates enough cytotoxic radicals to kill around 100% of cells under these in vitro conditions. We continued our investigations by using OLED-based light sources (Figure 4), to compare outcomes of LED and OLED light sources. The optimal output for OLED devices was 2 mW/cm2 and exposure time to kill cells was 45 and 60 minutes, which is equal to fluences of 5.4 and 7.2 J/cm2 respectively.
The direct comparison of OLED and LED exposures and their effect on C. fasciculata is shown in Figure 5. The effect of high-intensity light is more profound when similar doses are delivered to parasites. Evidently, it is due to the higher efficiency of the high density of light (50 mW/cm2), which generates a high concentration of radicals facilitating the killing and overcoming growth rate (doubled every 5-7 hours) of parasites [34].
In Table 2, we summarize our results with typical parameters, which have been used by authors in the current work for comparison when 25 µM MB is utilized.
Exposing Leishmania to MB photosensitizer at 50 µM or below only in the dark / in the absence of red light shows it has a detrimental effect on the cells in accordance with several other studies [20,22,24,28]. Our investigations showed no significant noticeable change in the viability of either L. major or C. fasciculata, which is consistent with the results from other groups as recently reviewed [35,36] and (Supplementary Data Table 1).
Discussion
The increasing prevalence of drug resistance in Leishmania over the past decades has become one of the primary causes of Leishmaniasis treatment failure, Horácio, et al. 2021 [37]. Thus, it is unsurprising that the use of PDT as a potential alternative to treat CL is gaining traction to prevent the widespread number of recurrence and unresponsive cases to drug therapies Cabral, et al. 2021 [9].
We have demonstrated that OLED and LED PDT can be effectively employed to kill C. fasciculata (a suitable non-infective to vertebrate model) and L. major in vitro even at low light intensities, i.e. at around 2 mW/cm2.
To improve the killing rates, thus the effectiveness of PDT for L. major, i.e. CL there is a need for higher output intensities for OLEDs, i.e. ~10 mW/cm2. In other words, the higher efficiency of high density of light generates a high concentration of radicals facilitating the killing of the parasites. In the future, it will be important to understand the mode of killing of these parasites caused by the PDT and if other photosensitizers provide a more efficient killing profile. Organic light-emitting diodes would be more efficient if it is brighter, they are already highly attractive for this practical application, where the flexibility of the material lends itself well to direct contact with the skin in any body location.
Conclusion
In this work, we have demonstrated that OLED and LED PDT can be effectively employed to kill C. fasciculata (a suitable non-infective to vertebrate model) and L. major in vitro even at low light intensities, i.e. at around 2 mW/cm2. Maybe unsurprisingly, due to their lifestyle C. fasciculata cells are more resistive than L. major. This may well be due to their lifestyle, as C. fasciculata are more likely to encounter harsher environmental conditions and therefore have mechanisms to cope with such stress. With further development, we foresee our light sources as wearable devices for the treatment of cutaneous Leishmaniasis, when either small or large skin areas are infected.
Funding
Royal Society Global Challenge grant CH160144.
- Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018 Sep 15;392(10151):951-970. doi: 10.1016/S0140-6736(18)31204-2. Epub 2018 Aug 17. PMID: 30126638.
- Monge-Maillo B, López-Vélez R. Therapeutic options for visceral Leishmaniasis. Drugs. 2013 Nov;73(17):1863-88. doi: 10.1007/s40265-013-0133-0. PMID: 24170666; PMCID: PMC3837193.
- van der Snoek EM, Robinson DJ, van Hellemond JJ, Neumann HA. A review of photodynamic therapy in cutaneous Leishmaniasis. J Eur Acad Dermatol Venereol. 2008 Aug;22(8):918-22. doi: 10.1111/j.1468-3083.2008.02805.x. Epub 2008 Jul 8. PMID: 18624853.
- Al-Bajalan MMM, Al-Jaf SMA, Niranji SS, Abdulkareem DR, Al-Kayali KK, Kato H. An outbreak of Leishmania major from an endemic to a non-endemic region posed a public health threat in Iraq from 2014-2017: Epidemiological, molecular and phylogenetic studies. PLoS Negl Trop Dis. 2018 Mar 1;12(3):e0006255. doi: 10.1371/journal.pntd.0006255. PMID: 29494612; PMCID: PMC5832184.
- Bezerra JMT, de Araújo VEM, Barbosa DS, Martins-Melo FR, Werneck GL, Carneiro M. Burden of Leishmaniasis in Brazil and federated units, 1990-2016: Findings from Global Burden of Disease Study 2016. PLoS Negl Trop Dis. 2018 Sep 6;12(9):e0006697. doi: 10.1371/journal.pntd.0006697. PMID: 30188898; PMCID: PMC6126835.
- Hamblin MR. Photodynamic Therapy for Cancer: What's Past is Prologue. Photochem Photobiol. 2020 May;96(3):506-516. doi: 10.1111/php.13190. Epub 2020 Jan 7. PMID: 31820824; PMCID: PMC7282978.
- Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010 May 12;110(5):2795-838. doi: 10.1021/cr900300p. PMID: 20353192; PMCID: PMC2896821.
- Sabino CP, Wainwright M, Ribeiro MS, Sellera FP, Dos Anjos C, Baptista MDS, Lincopan N. Global priority multidrug-resistant pathogens do not resist photodynamic therapy. J Photochem Photobiol B. 2020 Jul;208:111893. doi: 10.1016/j.jphotobiol.2020.111893. Epub 2020 May 11. PMID: 32446039.
- Dimmer J, Cabral FV, Sabino CP, Silva CR, Núñez-Montoya SC, Cabrera JL, Ribeiro MS. Natural anthraquinones as novel photosentizers for antiparasitic photodynamic inactivation. Phytomedicine. 2019 Aug;61:152894. doi: 10.1016/j.phymed.2019.152894. Epub 2019 Mar 13. PMID: 31054439.
- Cabral FV, Souza THDS, Sellera FP, Fontes A, Ribeiro MS. Towards effective cutaneous Leishmaniasis treatment with light-based technologies. A systematic review and meta-analysis of preclinical studies. J Photochem Photobiol B. 2021 Aug;221:112236. doi: 10.1016/j.jphotobiol.2021.112236. Epub 2021 May 31. PMID: 34090038.
- Verma NK, Dey CS. Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob Agents Chemother. 2004 Aug;48(8):3010-5. doi: 10.1128/AAC.48.8.3010-3015.2004. PMID: 15273114; PMCID: PMC478494.
- Vincent IM, Weidt S, Rivas L, Burgess K, Smith TK, Ouellette M. Untargeted metabolomic analysis of miltefosine action in Leishmania infantum reveals changes to the internal lipid metabolism. Int J Parasitol Drugs Drug Resist. 2013 Dec 5;4(1):20-7. doi: 10.1016/j.ijpddr.2013.11.002. PMID: 24596665; PMCID: PMC3940234.
- Fernandez-Prada C, Vincent IM, Brotherton MC, Roberts M, Roy G, Rivas L, Leprohon P, Smith TK, Ouellette M. Different Mutations in a P-type ATPase Transporter in Leishmania Parasites are Associated with Cross-resistance to Two Leading Drugs by Distinct Mechanisms. PLoS Negl Trop Dis. 2016 Dec 2;10(12):e0005171. doi: 10.1371/journal.pntd.0005171. PMID: 27911896; PMCID: PMC5135041.
- Chakravarty J, Sundar S. Drug resistance in Leishmaniasis. J Glob Infect Dis. 2010 May;2(2):167-76. doi: 10.4103/0974-777X.62887. PMID: 20606973; PMCID: PMC2889657.
- Cabral FV, Lian C, Persheyev S, Smith TK, Ribeiro MS, Samuel IDW. Organic Light‐Emitting Diodes as an Innovative Approach for Treating Cutaneous Leishmaniasis - Cabral - Advanced Materials Technologies - Wiley Online Library. Advanced materials technologies 2021: 2100395.
- Attili SK, Lesar A, McNeill A, Camacho-Lopez M, Moseley H, Ibbotson S, Samuel ID, Ferguson J. An open pilot study of ambulatory photodynamic therapy using a wearable low-irradiance organic light-emitting diode light source in the treatment of nonmelanoma skin cancer. Br J Dermatol. 2009 Jul;161(1):170-3. doi: 10.1111/j.1365-2133.2009.09096.x. Epub 2009 Mar 19. PMID: 19302071.
- Samuel IDW, Kulyk O, McNeill A, Moseley H, Ferguson J, Ibbotson S. Ambulatory photodynamic therapy of skin cancer, Photodiagnosis and Photodynamic Therapy. 2015; 12: 331.
- Quirk BJ, Brandal G, Donlon S, Vera JC, Mang TS, Foy AB, Lew SM, Girotti AW, Jogal S, LaViolette PS, Connelly JM, Whelan HT. Photodynamic therapy (PDT) for malignant brain tumors--where do we stand? Photodiagnosis Photodyn Ther. 2015 Sep;12(3):530-44. doi: 10.1016/j.pdpdt.2015.04.009. Epub 2015 May 8. PMID: 25960361.
- Brancaleon L, Moseley H. Laser and non-laser light sources for photodynamic therapy. Lasers Med Sci. 2002;17(3):173-86. doi: 10.1007/s101030200027. PMID: 12181632.
- Aureliano DP, Lindoso JAL, de Castro Soares SR, Takakura CFH, Pereira TM, Ribeiro MS. Cell death mechanisms in Leishmania amazonensis triggered by methylene blue-mediated antiparasitic photodynamic therapy. Photodiagnosis Photodyn Ther. 2018 Sep;23:1-8. doi: 10.1016/j.pdpdt.2018.05.005. Epub 2018 May 8. PMID: 29751117.
- Enk CD, Fritsch C, Jonas F, Nasereddin A, Ingber A, Jaffe CL, Ruzicka T. Treatment of cutaneous Leishmaniasis with photodynamic therapy. Arch Dermatol. 2003 Apr;139(4):432-4. doi: 10.1001/archderm.139.4.432. PMID: 12707088.
- Mang TS. Lasers and light sources for PDT: past, present and future. Photodiagnosis Photodyn Ther. 2004 May;1(1):43-8. doi: 10.1016/S1572-1000(04)00012-2. PMID: 25048063.
- Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther. 2004 Dec;1(4):279-93. doi: 10.1016/S1572-1000(05)00007-4. PMID: 25048432; PMCID: PMC4108220.
- Song D, Lindoso JA, Oyafuso LK, Kanashiro EH, Cardoso JL, Uchoa AF, Tardivo JP, Baptista MS. Photodynamic therapy using methylene blue to treat cutaneous Leishmaniasis. Photomed Laser Surg. 2011 Oct;29(10):711-5. doi: 10.1089/pho.2010.2915. Epub 2011 Jun 14. PMID: 21671755.
- Wallace FG. Flagellate Parasites of Mosquitoes with Special Reference to Crithidia fasciculata, Leger 1902. J Parasitol. 1943; 29:196-20.
- Kipandula W, Young SA, MacNeill SA, Smith TK. Screening of the MMV and GSK open access chemical boxes using a viability assay developed against the kinetoplastid Crithidia fasciculata. Mol Biochem Parasitol. 2018 Jun;222:61-69. doi: 10.1016/j.molbiopara.2018.05.001. Epub 2018 May 18. PMID: 29782894.
- Kogan BY. Nonlinear photodynamic therapy. Saturation of a photochemical dose by photosensitizer bleaching. Photochem Photobiol Sci. 2003 Jun;2(6):673-6. doi: 10.1039/b208616f. PMID: 12859152.
- Peloi LS, Biondo CE, Kimura E, Politi MJ, Lonardoni MV, Aristides SM, Dorea RC, Hioka N, Silveira TG. Photodynamic therapy for American cutaneous Leishmaniasis: the efficacy of methylene blue in hamsters experimentally infected with Leishmania (Leishmania) amazonensis. Exp Parasitol. 2011 Aug;128(4):353-6. doi: 10.1016/j.exppara.2011.04.009. Epub 2011 May 7. PMID: 21575635.
- Pinto JG, Martins JFS, Pereira AHC, Mittmann J, Raniero LJ, Ferreira-Strixino J. Evaluation of methylene blue as photosensitizer in promastigotes of Leishmania major and Leishmania braziliensis. Photodiagnosis Photodyn Ther. 2017 Jun;18:325-330. doi: 10.1016/j.pdpdt.2017.04.009. Epub 2017 Apr 27. PMID: 28457848.
- Pinto JG, Soares CP, Mittmann J. Assessment of Leishmania major and Leishmania braziliensis promastigote viability after photodynamic treatment with aluminum phthalocyanine tetrasulfonate (AlPcS4). J Venom Anim Toxins incl Trop Dis. 2011; 17: 300-307.
- Nesi-Reis V, Navasconi TR, Lera-Nosone DSSL, Oliveira EL, Barbosa PM, Caetano W, Silveira TGV, Aristides SMA, Hioka N, Lonardoni MVC. Phototoxic effect of aluminium-chlorine and aluminium-hydroxide phthalocyanines on Leishmania (l.) amazonensis. Photodiagnosis Photodyn Ther. 2018 Mar;21:239-245. doi: 10.1016/j.pdpdt.2017.12.008. Epub 2017 Dec 21. PMID: 29275066.
- Lian C, Piksa M, Youshida K, Persheyev S, Pawlik KJ, Matczyszyn K, Samuel IDW. Flexible organic light-emitting diodes for antimicrobial photodynamic therapy. NPJ Flexible Electronics. 2019; 18: 1-6.
- Mikus J, Steverding D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol Int. 2000 Jan;48(3):265-9. doi: 10.1016/s1383-5769(99)00020-3. PMID: 11227767.
- Sazhnev V, DeKrey GK. The growth and infectivity of Leishmania major is not altered by in vitro exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. BMC Res Notes. 2018 Sep 4;11(1):642. doi: 10.1186/s13104-018-3759-x. PMID: 30180875; PMCID: PMC6122646.
- Pinto JG, Marcolino LM, Ferreira-Strixino J. Photodynamic activity of Photogem® in Leishmania promastigotes and infected macrophages. Future Microbiol. 2021 Jan;16(2):95-106. doi: 10.2217/fmb-2020-0019. Epub 2021 Jan 18. PMID: 33459574.
- Varzandeh M, Mohammadinejad R, Esmaeilzadeh-Salestani K, Dehshahri A, Zarrabi A, Aghaei-Afshar A. Photodynamic therapy for Leishmaniasis: Recent advances and future trends. Photodiagnosis Photodyn Ther. 2021 Dec;36:102609. doi: 10.1016/j.pdpdt.2021.102609. Epub 2021 Oct 31. PMID: 34728420.
- Horácio ECA, Hickson J, Murta SMF, Ruiz JC, Nahum LA. Perspectives From Systems Biology to Improve Knowledge of Leishmania Drug Resistance. Front Cell Infect Microbiol. 2021 Apr 30;11:653670. doi: 10.3389/fcimb.2021.653670. PMID: 33996631; PMCID: PMC8120230.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
