Global Journal of Infectious Diseases and Clinical Research
Clinical profile of Dengue infection at a center in north Karnataka, India
Adnan Imam1 and Prashanth ED2*
Cite this as
Imam A, Prashanth ED (2019) Clinical profile of Dengue infection at a center in north Karnataka, India. Glob J Infect Dis Clin Res 5(1): 006-009. DOI: 10.17352/2455-5363.000022Dengue is one of the most common arbovirus infection worldwide, which is a vector borne disease caused by the bite of Ades Aegypti mosquito. Symptoms of the infected individuals have a very broad range of presentation having similarities with other infections like malaria and influenza like illness etc. It is a retrospective study conducted at a centre in north Karnataka with the sampling method of quota or sequential sampling with inclusion of 121 cases all seropositive for dengue antigen or antibodies or both in the monsoon season from july 2018 till November 2018. The complete blood picture and liver enzymes like SGPT and SGOT were assessed during the period of the infected individuals. Leucopoenia is taken below 4000 cells/cumm, thrombocytopenia is taken below 100000 per cumm and liver enzymes were evaluated and graded as per the CTCAE guidelines version 4.0. The two tailed T Test was used to determine the significant change in the SGOT and SGPT liver enzymes. The symptoms ranged from mild to severe ones like fever, headache, nausea, vomiting, abdominal pain, generalised body ache (myalgia), cough, Rash, retro bulbar pain, bleeding, hypotension, signs of plasma leakage, leucopoenia, thrombocytopenia, SGOT (serum glutamic oxaloacetic transaminase) or AST (Aspartate aminotranferase) and SGPT (Serum glutamic pyruvic transaminase) or ALT (Alanine Transaminase), hypotension (blood pressure of 90/60 mm of Hg or lower), pleural effusion and ascites. 1.7% of the individual even showed seropositivity but didn’t have any episode of fever and elevation of SGOT was significantly raised with respect to SGPT which was nearly within normal range for nearly half of the infected patients. This indicates that muscle injury is more significant with respect to the liver injury during the course of the disease. Negligible number of individual landed up in complications like Dengue Hemorrhagic fever or dengue shock syndrome if prompt and proper management of the disease is done. It was also seen that the affected individuals were more from the age group of 16 years to 37 years, the cause for which still need to be explained on a bigger population size.
Introduction
Dengue is the most common arbovirus infection worldwide, with transmission occurring in at least 128 countries and around 4 billion individuals are at risk [1]. The dengue cases reported to WHO has increased regularly as an average of less than a thousand cases every year worldwide in the 1950s to more than 3 million cases in 2013 [2-5]. Dengue is a vector borne disease, caused by dengue virus having serotypes 1-4 and is one of the most important arboviral infections in the subtropical and tropical regions where environmental conditions favour the development and proliferation of Aedes Aegypti, the principal vector of this zoonosis [6,7].
The first case was reported in Madras (now Chennai in 1780, and first outbreak happened in Calcutta (now Kolkata) in 1963 and further outbreaks were also reported in the different parts of India [8,9], Aedes aegypti can breed in polluted water or small collections of water such as flower vases or coconut shells [10]. Eggs can survive for long periods, as they are capable of withstanding desiccation. Inadequate wastewater drainage facilitates or improper disposal of garbage, both consequences of unplanned urbanisation, may be responsible for high mosquito densities in endemic areas, during rainy season there is significant increase in the mosquito larval population and this may be the reason that epidemics of the disease coincide with the rainy season [11]. After biting an infected human, dengue viruses enter an adult female mosquito. The virus first replicates in the mid-gut, reaches the haemocoele and haemolymph, and then gains access to different tissues of the insect. After viral replication in the salivary glands, the infected mosquito can transmit the virus to another human [12].
Dengue infections vary in severity, ranging from influenza-like self-limiting illness to lifethreatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) which, if left untreated, are associated with mortality as high as 20% [13].
Symptomatic dengue infections have a very broad range of presentation and severity and near about 70% of patients do not take treatment from health care professionals and want to treat themselves on their own [14]. Individuals even who are seen by a healthcare professional, the clinical presentation of the disease shares similarities with 12 major pathogens, which makes misdiagnosis very common, particularly in areas where there is a high incidence of febrile illnesses [15]. The various manifestations of dengue may not have a clear line of demarcation: apart from the classic features, reports of rare presentations have recently become more frequent [16,17].
The aim of the study is to assess the clinical presentation of the disease with varying degree of symptoms from a milder to life threatening ones with biochemical and haematological parameters alterations in the diseased state.
Material and Methods
This was a retrospective study conducted among 121 patients suffering from Dengue infection visiting the KBN Teaching and General Hospital in the period of 5 months i.e., the monsoon and the autumn season (highest prevalent seasonal fluctuation in dengue). The sampling method used was that of sequential or quota sampling so as to include each and every patient suffering from dengue visiting the hospital as the seasonal prevalence and periodicity of dengue was impractical to assume and base the study on; owing to the lack of previous such studies in the area. We included all the patients who were seropositive showing NS 1 antigen for dengue (Non structural protein 1) or IgM antibodies in their serum or both as per the previous medical records above the age of 12 years. All the history and clinical details including the symptoms as mentioned in the patient record file were obtained from the Medical Record Section of the Institute from July 2018 till November 2018 (As per the suitable medical ethic, In the process no data which would harm the patient’s medical and social aspects of life nor the identity of the patient was revealed). The entire sample’s liver function test profile was taken into consideration as the main criteria to assess and to show the impact of disease and the subsequent data was graded as per the CTCAE (Common Terminology Criteria for Adverse Events) guidelines [18]. The blood picture of the sample was also commented upon. The statistical test used to show the significance of the comparison between the two primary liver parameters, i.e., SGOT and SGPT and the relationship between the two in respect to the progression of the disease was elaborately and significantly proved during the course of the study using Two Tailed T Test. All the statistical analyses were done in Statistical Package for Social sciences (SPSS) version 22.0.
Results
We analysed the data of 121 patients out of which 76 (62.8%) were males and 45 (37.2%) were females. The male: female ratio was 1.68:1. The mean age of the patients in the study was 24.59 with a minimum of 12 yrs to maximum of 80 years.
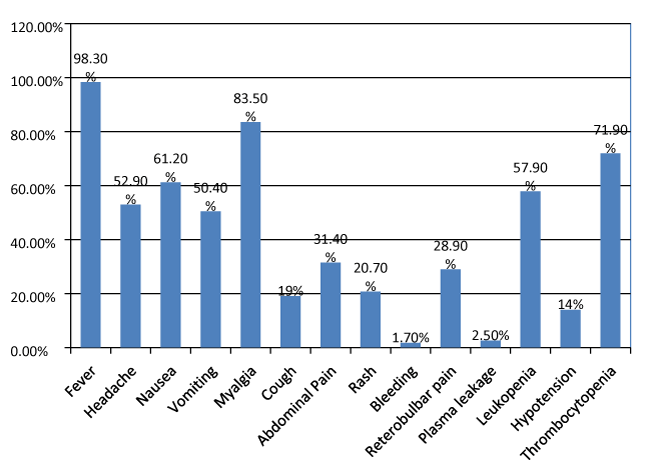
The main presenting symptoms and laboratory parameters taken into account are fever, headache, nausea, vomiting, abdominal pain, generalised body ache (myalgia), cough, Rash, retro bulbar pain, bleeding, hypotension, signs of plasma leakage, leucopoenia, thrombocytopenia, SGOT (serum glutamic oxaloacetic transaminase) or AST (partate aminotranferase) and SGPT (Serum glutamic pyruvic transaminase) or ALT (Alanine Transaminase). Hypotension was considered with a blood pressure of 90/60 mm of Hg or lower. The rise in level of liver enzymes were graded as per the CTCAE guidelines as Grade 1 to 4. Thrombocytopenia was considered with platelet count of < 100,000/µl, Leucopoenia with values <4000/µl. Plasma leakage was assessed based on the presence of any pleural effusion and presence of ascites. Out of 121 individuals 119 (98.3%) presented with fever, 64 (52.9%) individuals had headache, 74 (61.2%) had nausea, 61 (50.4%) had vomiting, 101 (83.5%) had myalgia, 23 (19%) had cough, 38 (31.4%) had abdominal pain, 35 (28.9%) had retro orbital pain, 25 (20.7%) had rashes, 2 (1.7%) had spontaneous bleeding, 17 (14%) had hypotension, 70 (57.9%) had leucopoenia, 87 (71.9%) had thrombocytopenia. The data has been shown in the bar graph as well for better view of the presenting signs and symptoms in figure 1.
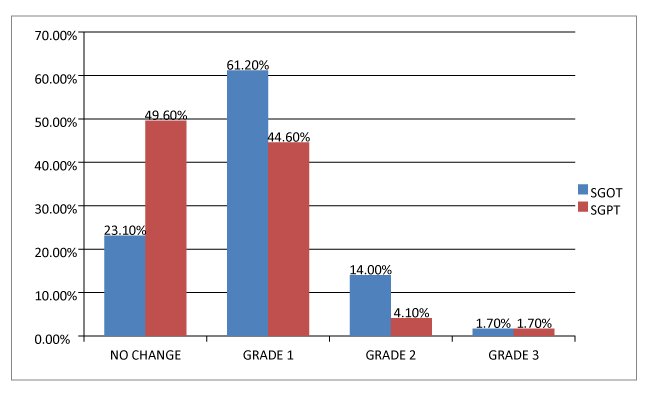
The hepatic damage reflected by the elevated ALT and AST was assessed and graded as described before. Out of total 121 individuals the ALT (SGPT) levels of 60 (49.6%) were not altered and within the normal range, 54 (44.6%) had grade 1 elevation, 5 (4.1%) had grade 2 elevation and 2 (1.7%) had grade 3 elevation. The AST (SGOT) levels of 28 (23.1%) had no changes in their enzymes level and it was within normal range, 74 (61.2%) had grade 1 changes, 17 (14%) had grade 2 changes and 2 (1.7%) had grade 3 changes. The grade 4 changes in the liver enzymes were not noted either for AST nor ALT. It was interesting to note that SGOT levels were altered more than SGPT levels in the scope of the study as per the data in the figure 2.
On statistical analyses with Two Tailed T Test, it was seen that upon comparison between the two sets of data, i.e., SGOT and SGPT levels, in pertinence to the scope of the study which was to prove that SGOT levels vary more significantly and is more reflective of dengue as far as liver enzymes are concerned, it was found that the findings were significant at 95 %(P<0.50) confidence level at 0.236.
Discussion
As already been discussed that a patient with dengue infection may manifest with various symptoms and sign which overlaps with many other condition causing acute febrile illness and can be misdiagnosed [19]. During the study we saw that the affected individuals are mainly between the age group of 16 years to 37 years, the older age group was less affected in numbers which as already been seen in other studies too, but it has been also seen that the young adult patients of dengue had a less stay in hospital with respect to the elderly who needs longer hospitalization [20]. However, maybe aging is related with significant changes in the adaptive humoral and cell mediated immunity as well as adaptive humoral immunity [21]. During the assessment of the data we noticed that out of 121 individuals 2 (1.7%) individuals they did not even complaint of high grade fever, but had thrombocytopenia and leucopoenia with SGOT derangement which may have pointed towards the suspicion of dengue infection. This shows that patient may present with wide range of symptoms and signs even without a history of fever in very rare cases, without thrombocytopenia or leucopoenia as well.
Other important thing to be noticed that out of all 121 individuals we did not got a single person with any comorbidities like hypertension, diabetes mellitus or obesity etc. The reason for this is still to be searched for and not much study has been done in India and other places to see the presentation and outcome of the dengue infection with comorbidities.
One more important thing to be noted is that out of all the patients only 14 had spontaneous bleeding and only 2 patients fitted into the definition of dengue hemorrhagic fever as per WHO [13]. There was no mortality in the study data, this clearly shows and support other studies that early diagnosis helps for a better outcome with early treatment and prevent the patient to land up in complications like dengue hemorrhagic fever and dengue shock syndrome, and experience a complete recovery without complications [22-24].
We also found that the thrombocytopenia is more common with respect to the leucopoenia which is also present in 57.9% cases. The rise in SGOT is more with respect to the SGPT levels which have already been shown in various other studies, nearly all the studies showed raised ALT and AST [25-28]. Our study shows that SGPT rise was not seen in nearly half of the patients that is 49.6% with a raise in the SGOT levels. So we can clearly say that the hepatic damage is there but the raised SGOT, points damage more towards the skeletal muscle system due to inflamed muscle cells.
Conclusion
So to conclude the study with different aspects we got points that the affected individuals were mainly from the age group of 16 to 37 years of age with a mean of 24.59 with a standard deviation of ±11.985, explanation of which is still to be researched. The range of symptoms varies considerably from fever, myalgia to life threatening situations like dengue hemorrhagic fever or dengue shock syndrome if not treated on time. The patients may even present with abdomen pain and on routine investigations we can see thrombocytopenia and leucopoenia even in a afebrile presentation. SGOT rise is more significant with respect to the rise of SGPT because some patients will not show SGPT elevation and point towards the help in diagnosing the diseased condition sometimes in early stages and gives a query to rule out dengue infection in acute febrile illness.
- Brady OJ, Gething PW, Bhatt S (2012) Refi ning the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6: e1760. Link: https://tinyurl.com/y26bgmzu
- WHO (2015) Dengue Net.
- WHO (2015) Vector borne and neglected tropical diseases. SEARO.
- PAHO (2014) Number of reported cases of dengue and severe dengue in the Americas, by country. Link: https://tinyurl.com/y4ekbupy
- WPRO (2015) Annual dengue data in the Western Pacifi c region. Link: https://tinyurl.com/y4ekbupy
- Halstead SB (2007) Dengue. Lancet 370: 1644–1652. Link: https://tinyurl.com/y6b3gnvf
- Mustafa MS, Rasotgi V, Jain S (2015) Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med J Armed Forces India 71: 67–70. Link: https://tinyurl.com/y644kd7j
- Ramakrishnan SP, Geljand HM, Bose PN (1964) The epidemic of acute haemorrhagic fever, Calcutta, 1963; epidemiological inquiry. Indian J Med Res 52: 633-650. Link: https://tinyurl.com/y5cv6gs7
- Chaturvedi UC, Nagar R (2008) Dengue and dengue haemorrhagic fever: Indian perspective. J Biosci 33: 429-441. Link: https://tinyurl.com/y49phw8a
- Vezzani D, Schweigmann N (2002) Suitability of containers from different sources as breeding sites of Aedes aegypti (L) in a cemetery of Buenos Aires City, Argentina. Mem Inst Oswaldo Cruz 97: 789-792. Link: https://tinyurl.com/y46dj3sx
- Thavara U, Tawatsin A, Chansang C (2001) Larval occurrence, oviposition behavior and biting activity of potential mosquito vectors of dengue on Samui Island, Thailand. J Vector Ecol 26: 172-180. Link: https://tinyurl.com/y3mafejm
- Malavige GN, Fernando S, Fernando DJ (2004) Dengue viral infections. Postgrad Med J 80: 588-601. Link: https://tinyurl.com/y5pstnj8
- WHO (2009) Dengue Guidelines for Diagnosis, Treatment, Prevention and Control WHO. Link: https://tinyurl.com/yygwd9ld
- Bhatt S, Gething PW, Brady OJ (2013) The global distribution and burden of dengue. Nature 496: 504-507. Link: https://tinyurl.com/y4brg4hq
- Simmons CP, Farrar JJ, van Vinh Chau N, Wills B (2012) Dengue. N Engl J Med 2012; 366: 1423-1432. Link: https://tinyurl.com/y4xcvyts
- Gulati S and Maheshwari A (2007) Atypical manifestations of dengue. Trop Med Int Health 12: 1087-1095. Link: https://tinyurl.com/y3d9qe44
- Misra UK, Kalita J, Syam UK, Dhole TN (2006) Neurological manifestations of dengue virus infection. J Neurol Sci 244: 117-122. Link: https://tinyurl.com/y6ytlfan
- National Cancer Institute (2010) Common Terminology Criteria for Adverse Events (CTCAE). Link: https://tinyurl.com/y6xrk974
- Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, et al. (2006) Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol 100: 363-370. Link: https://tinyurl.com/y6jlabpd
- Ching-Chi Lee, Hsiang-Chin Hsu, Chia-Ming Chang (2013) Atypical presentations of dengue disease in the elderly visiting the ED. American Journal of Emergency Medicine 31: 783–787. Link: https://tinyurl.com/y2c3uxn3
- Grubeck-Loebenstein B, Wick G (2002) The aging of the immune system. Adv Immunol 80: 243-284. Link: https://tinyurl.com/y6y6atc9
- Guzman MG, Kouri G (2003) Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol 27: 1-13. Link: https://tinyurl.com/yxmcj46a
- Guzman MG, Kouri G (2002) Dengue: an update. Lancet Infect Dis 2: 33-42. Link: https://tinyurl.com/yy73fg3s
- da Fonseca BA, Fonseca SN (2002) Dengue virus infections. Curr Opin Pediatr 14, 67-71. Link: https://tinyurl.com/y22xq4pe
- Sharma S, Sharma SK (1998) Clinical profile of DHF in adults during 1996 outbreak in Delhi, India. Dengue Bulletin 22: 20-27. Link: https://tinyurl.com/y2byp67l
- Daniel R, Rajamohanan, Philip AZ (2005) A study of clinical profile of dengue fever in Kollam, Kerala, India. Dengue Bulletin 29: 197-202. Link: https://tinyurl.com/yylcxolq
- Itha S, Kashyap R, Krishnani N, Saraswat VA, Choudhuri G, et al. (2005) Profile of liver involvement in dengue virus infection. Natl Med J India 18: 127-130. Link: https://tinyurl.com/y4rn6o7e
- Kuo CH, Tai DI, Chang-Chein CS, Lan CK, Chiou SS, et al. (1992) Liver biochemical tests and dengue fever. Am J Trop Med Hyg 47: 265-270. Link: https://tinyurl.com/y5fy9vue
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
