Global Journal of Infectious Diseases and Clinical Research
Toxocariasis and Public Health: An Epidemiological Review
Anunobi Toochukwu Joy1, Okoye Ikem Chris2 and Nwosu Chigozie Godwin2*
2Parasitology and Biomedical Diseases Research Unit; Zoology and Environmental Biology Department, University of Nigeria Nsukka, Enugu State, Nigeria
Cite this as
Joy AT, Chris OI, Godwin NC (2017) Toxocariasis and Public Health: An Epidemiological Review. Glob J Infect Dis Clin Res 3(1): 028-039. DOI: 10.17352/2455-5363.000016An epidemiological review of toxocariasis aimed to underpin its prevalence, proclivity and prognosis was undertaken. Human toxocariasis constitutes one of the most common parasitic infections worldwide, which is more prevalent in developing and tropical countries. Human infection is caused by ingesting the eggs which were shed in the faeces of the definite dog or cat host. There is a range of clinical manifestations of toxocariasis in human, but the two classical clinical syndromes often described are visceral and ocular larva migrans. The clinical signs and complications which result from infection with this parasite are mostly dependent on the number and migration locations of Toxocara larvae. Visual identification of larvae in tissues and organs is the gold standard for toxocariasis diagnosis in human, while an enzyme linked immunosorbent assay detecting Imunoglobulin-G antibodies against Toxocara excretory-secretory antigen is the reference test for immunodiagnosis. In human, loss of vision, hypereosinophilia, encephalitis and problems involving the liver, lung and the central nervous system are the most important complications. Poor hygiene, poverty and lack of education can exacerbate the exposure to Toxocara infection. Albendazole is the treatment of choice for toxocariasis. Conclusively, the present review recommends that regular stool examination and frequent chemotherapy of pets can be effective in reducing the egg number deposited in soil; reducing the number of pet animals or limiting contacts of small children with them and good hygiene practices will limit transmission of toxocariasis.
Introduction
Toxocariasis is the clinical term applied to infection in the human host with either Toxocara canis (Werner, 1782) or Toxocara cati (Schrank, 1788). Both of these are ascarid nematodes in the order Ascaridida, superfamily Ascaridiodea, and family Toxocaridae. The definitive hosts of T. canis and T. cati are the domestic dog and cat respectively. They can be found in different parts of the body, including the liver, heart, lungs, brain, muscle or eye [1]. The clinical signs and complications which result from infection with this parasite are mostly dependent on the number and migration locations of Toxocara larvae [2]. In human, loss of vision, hypereosophilia, encephalitis and problems involving the liver, lung and the central nervous system are most important complications caused by this parasite [2]. Other names applied to toxocariasis include Weingarten’s disease, Frimodt-Møller’s syndrome, and eosinophilic pseudoleukemia [3], and also nematode ophthalmitis, toxocaral disease, toxocarose, and covert toxocariasis [4]. T. canis and T. cati are distributed worldwide [5], which is more prevalent in tropical, developing countries and poor communities [6]. Human quest for surrounding ourselves with various domestic animals, particularly cats and dogs, has ensured a worldwide distribution for toxocariasis [7,8]. Poor hygiene, poverty and lack of education can exacerbate the exposure to Toxocara spp (Quattrocchi et al., 2012).
Etiologic Agents, Hosts And Distribution
Aetiologic Agents
Zoonotic Toxocara species include Toxocara canis, T. cati, and possibly T. vitulorum and T. pteropodis. These nematode parasites all belong to the family Toxocaridae. T. canis is generally thought to be more important than T. cati in human disease. T. cati has been implicated particularly in ocular toxocariasis [9,10]. T. vitulorum infection is thought to be a low level zoonosis mainly affecting children in the tropics. T. pteropodis, a nematode of fruit bats, was implicated in an outbreak of hepatitis associated with feces-contaminated fruit in Palm Island, Australia [11]. This association has been questioned by some authors.
Two new species have recently been identified: T. malayasiensis [12], in the domestic cat and T. lyncus in caracals [13]. The zoonotic potential of these two organisms is unresolved [11,14,15]. Toxocara canis (Werner, 1782) and Toxcara cati (Schrank, 1788) are common intestinal roundworms of canids and felids, respectively that are often implicated in human toxocariasis.
Historical background: Werner described a parasitic nematode in dogs in 1782 which he named Ascaris canis. Johnston however identified what Werner had described as a member of the genus Toxocara and established by Stiles in 1905. Fülleborn speculated that T canis larvae might cause granulomatous nodules in humans. In 1947, Perlingiero and Gyorgy described the first case of what was probably toxocariasis. Their patient was a 2-year-old boy from Florida who had classical symptoms and esoinophilic necrotizing granulomas [3]. In 1950, Campbell-Wilder was the first to describe toxocariasis in humans; she published a paper describing ocular granulomas in patients with endophthalmitis, Coat’s disease, or pseudoglioma. Two years later, Beaver et al published the presence of Toxocara larvae in granulomas removed from patients with symptoms similar to those in Wilder’s patients [16,7,9].
Habitat: The eggs of T. canis are excreted in the feces of an infected canid host. The embryonated eggs can live in the feces for up to three weeks. The feces are often deposited in soil or sandy areas. A host must ingest the eggs for the life cycle to continue. If ingested, the new habitat becomes the internal organs of the host. The gut is the first area T. canis larvae reside. If the host has not been previously infected, hatched juveniles go throught the circulation to the lungs, then back to the gut. If in a canid host, they take up residence in the intestine and develop into adults. If hosts have been previously “immunized” junveniles go to the body tissues and become dormant as if they were in a paratenic host. Often the infectious larvae stay in the mammary glands until a pregnancy where they are passed on to a nursing pup. If in a human or other non-canid host the larvae will wonder throughout the organs. These wandering larvae are called visceral larva migrans. They may travel to the eyes, lungs, brain, heart, muscles, liver, and other organs. Here they do not develop further but can cause severe local reactions [17,18].
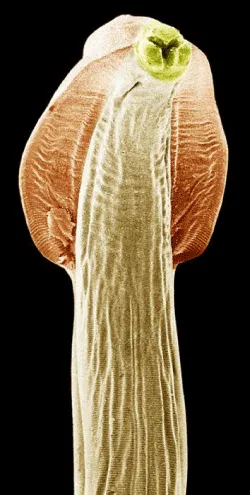
Morphology of Toxocara species: T. canis and T.cati are ascarid nematodes [19,7], in the order Ascaridida, superfamily Ascaridiodea, and belong to the family Toxocaridae [7]. Toxocara canis is smaller than most of the other species in the family Ascarididae. It has a complete gut in the form of a simple tube. It is a “round worm” implying the shape of the outer layer to be round (if seen in a cross section). Depending on the host the worm gets into, T. canis will have different number of larval stages. Most worms have three larval stages before becoming infective. The larvae of T. canis are 290 to 350 μm by 18 to 21 μm. The diameter of the larvae of T. cati is somewhat smaller. The morphology of the adult worms resembles that of Ascaris lumbricoides, but they are much smaller. Toxocara canis is dioecious having morphology distinctly different for the male and female. Toxocara adult parasites are large, pink, roundworms, measuring 6.5 to 10 cm long for the females and 4 to 6 cm long for the males [20]. The male’s posterior end is curved ventrally and the tail is bluntly pointed which distinguishes it from straight- tailed female [7]. The male has a single tubular testis. He also has simple spicules, which allows for direct sperm transfer. The female worms are generally around 6.5 cm but can be as long as 15 cm long. In the female the vulva is about one-third the body length from the anterior end. The ovaries are very large and extensive. The uteri contain up to 27 million eggs at a time.
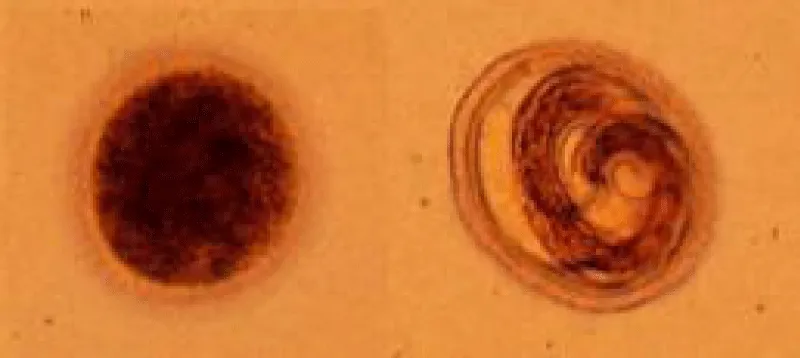
Both males and females have three prominent lips. Each lip has a dentigerous ridge. The lateral hypodermal cords are visible with the naked eye. No gubernaculums is present. In both sexes there are prominent cervical alae [21]. The eggs are brownish and almost spherical. The brownish eggs of T. canis and T. cati measure approximately 85 by 75 μm and 75 by 65 μm, respectively. The eggs are almost spherical, larger than those of A. lumbricoides and unembryonated when laid. The eggs are embryonated when laid and have surficial pits. These eggs are very resistant to various weather and chemical conditions [22,23] (Figures 1,2).
Communication and perception: Nematodes within the Secernentea have phasmids, which are unicellular glands. Phasmids likely function as chemoreceptors. Females may produce pheromones to attract males. Nematodes in general have papillae, setae and amphids as the main sense organs. Setae detect motion (mechanoreceptors), while amphids detect chemicals (chemoreceptors) [24,23].
Food habits: The location of T. canis in hosts is in the small intestine. There they feed on intestinal contents. The adults have a specialized anaerobic metabolism. This specialized metabolism gives the adult worms an extra ATP. Adult T. canis worms are very host specific. Pharyngeal glands and intestinal epithelium produce digestive enzymes to feed on the hosts’ body fluids. Extracellular digestion begins within the lumen and is finished intracellularly (Barnes, 1987; Roberts and Janvory, 2000) [24,23].
Host
Definitive hosts: Toxocara canis is transmitted predominantly among canids (dogs, foxes, wolves and coyotes) and T. cati by felids through a wide variety of routes. These include vertical transmission, transplacental (not cats) and/or trans-mammary (lactogenic) as well as horizontal transmission through the ingestion of embryonated eggs from the environment or ingestion of larvae via vertebrate and/or invertebrate paratenic hosts [25]. It is the ability of T. canis to survive for many years in the tissues of a substantial number of vertebrate species, as well as to develop to sexual maturity in the intestinal tract of its definitive canid hosts, especially dogs, that has facilitated its global distribution. Pups are infected in utero by reactivated, somatic larvae of T. canis from the mother from day 42 of gestation. This efficient trans-placental infection route results in egg excretion 16 days after parturition [26]. More limited, lactogenic transmission continues to occur for 5 weeks. Kittens are infected by vertical, lactogenic transmission of T. cati and commence fecal egg excretion 7 days after birth [25,27]. Once infected, pups shed millions of eggs per day into the environment, depending on the intensity of T. canis infection and host immune status (Glickman and Schantz, 1981). Dogs and other canids also become infected by ingesting embryonated eggs from the environment. Toxocara canis undergoes a tracheal migration and has a prepatent period of 4–5 weeks. In cats, the prepatent period is 8 weeks.
Toxocara canis infections can be acquired at any age, although adult worm infections are generally less common in dogs less than 6 months of age, and fecal egg counts are much lower than in pups [28,29]. Paradoxically, low levels of egg exposure are more successful for the establishment of patent infections in juvenile/adult dogs than large numbers of eggs. This finding may have long term implications for control programs [25]. Toxocara spp. do not usually cause pathological changes in definitive host species, although high infection intensities in transplacentally infected puppies can result in a pot-bellied appearance, failure to thrive and, in some instances, death Lloyd and Morgan, 2011 [25].
Paratenic hosts: Mammals (rodents, lagomorphs, birds and domestic livestock) are susceptible to infection by embryonated eggs containing infective L3s, which migrate to the tissues, where they undergo no further development and remain infective for up to 7 years [30,31,32]. L3s may also be found in a range of invertebrate hosts including earthworms. Dogs which ingest paratenic hosts can develop adult worms but there is no tracheal migration [25]. L3s ingested by omnivorous or carnivorous paratenic hosts can migrate to the tissues of a new paratenic host. Ingestion of paratenic hosts plays an important role in T. cati infection for adult cats. Transmission of the parasite from infected rodents may be facilitated by behavioral changes induced in rodents infected with T. canis, which may impair survival and fitness relative to the intensity of infection [33]. Post mortem examination of rats (Rattus norvegicus) which had been experimentally infected with T. canis revealed L3s in muscle, eye, liver, kidney, brain and lung [34]. The same situation may apply to T. cati.
Toxocara transmission in definitive host: There are four ways of infection or transmission from one host to the next. Toxocara canis can be transmitted to nursing pups by transmammary transmission from larvae that were in their mother’s milk. This is the least common form of transmission. The ingested milk containing the infected stage three larvae goes directly to the newborns’ small intestine. Here the larvae develop directly into adults. Prenatal transmission is the second form of obtaining T. canis. Infected pups are born with larvae already in their bodies. Stage two larvae (not yet infective) migrate from tissue in the pregnant mother. The larvae travel across the placenta and through the umbilical cord to the fetal liver. The larvae remain in the liver until birth. Once the pup is born the larvae resume their migration to the lungs of the newborn. Another form of transmission is direct transmission. In this form of transmission the new host directly ingests the eggs from the feces of another infected canid. Toxocara canis larvae migrate from the gut (where they were ingested) to the small intestine of newborns. Toxocara canis also migrates to the lungs from the intestines. They move up the bronchial tree and the trachea to the pharynx, here they are swallowed by the newborn to finally reach the intestine. In the intestine the larvae mature. Only a small number of the larvae infecting the host actually undergo migration to the trachea. The majority of larvae continue to migrate through the lungs and the pulmonary veins of the host. The larvae migrate to the heart and there they are distributed to the somatic tissues via the peripheral circulation. The conditions for prenatal and transmammary transmission to the pups is set up by these somatic migration conditions. The last form of transmission is paratenic host transmission. Many paratenic hosts contain the non-infective larvae of T. canis. When one of these animals is eaten by another host transmission takes place. A good example is when a dog eats an infected mouse. In the case of ingesting an infected paratenic host no further migration takes place in the dog since the requirement for the life cycle migration is already satisfied in the paratenic (i.e. mouse) host [18,23].
Reproduction and development of Toxocara in definitive host: Females may produce a pheromone to attract males. The male coils around a female with his curved area over the female genital pore. The gubernaculum, made of cuticle tissue, guides spicules which extend through the cloaca and anus. Males use spicules to hold the female during copulation. Nematode sperm are amoeboid-like and lack flagella. The adult worm remains in the intestine and produces an enormous number of eggs (up to 200,000 unembryonated eggs each day). Eggs begin to appear in the canid feces fifth week post infection [35,15], and up to eight weeks in T. canis. Under optimum conditions, eggs will embryonate and become infective within 6 weeks, but this can be delayed for several months’ lower temperatures [36].
Toxocara canis has a complex life cycle. Similar to other nematodes, T. canis egg is not infectious immediately when it leaves the definitive host until it has developed into ensheathed L3, infectious stage. When ingested by a canine host, T. canis larvae hatch in the small intestine, burrow through the intestinal mucosa, enter the bloodstream and travel via the liver to the lungs [35]. From here, the larvae either migrate up the trachea where they are swallowed and returned to the small intestine to develop into adult worms or undergo somatic migration and enter a wide range of tissues including the liver, lungs, heart, brain and muscle [15]. The larvae can become mobilised from the tissues and migrate across the placenta infecting puppies in utero, leading to tracheal migration in the pup and eggs being shed in the faeces 2–3 weeks after birth, or they can migrate to the mammary glands and infect puppies during lactation. There is no transplacental transmission with T. cati [15] (Figures 3-5).
Distribution of Toxocara
Geographic range and distribution of Toxocara: Toxcocara has a worldwide distribution [15]. It is prevalent in all locations that have domestic dogs, puppies, and other canids. Toxocara is also found in places that have other various mammals such as mice, pigs, birds, and foxes, but these hosts are only paratenic hosts. Hosts are terrestrial mammals and therefore T. canis is mainly found in terrestrial terrain [17]. The eggs of these species occur in 2-88% of soil samples collected in various countries and regions. T. vitulorum is found mainly in the tropics; cases have been reported from 50° north of the equator to 40° south. T. vitulorum is present in the U.S. but the prevalence of infection is low. The high ambient temperatures and humidity of the tropics favor the transmission of Toxocara species. In Iceland, where dogs have been banned since the 1940s, visceral larva migrans is very rare and 0 of 300 human adults had antibodies to Toxocara spp.
Soil contamination of Toxocara spp, eggs survival and dispersal: In Nigeria, reports on soil contamination by Toxocara eggs have been reported in Nsukka, Enugu state [37]; Calabar [38]; Kaduna [39] and other places. It is estimated that the contamination of soil with Toxocara eggs may be more than the 90% of the investigated areas worldwide Joanna, 2015 [40]. This is explained by the fact that mature eggs of ascarids can survive in contaminated soil even in harsh conditions (e.g. they may resist to chemicals, broad temperature ranges and several degrees of moisture), thus are available for ingestion at any time by susceptible hosts [7]. Also, viability and infectivity of environmental larvated eggs persist for years, thus explaining the high number of chances that dogs have of becoming infected and the difficulties in controlling these intestinal parasitoses [41]. Moreover, larvated eggs of T. canis are an efficient environmental source of infection for various animals, which act as paratenic hosts. These animals greatly contribute to maintaining the biological cycle of toxocariasis everywhere. In fact, dogs can become infected by Toxocara by ingesting tissues of invertebrates (e.g. earthworms), ruminants (e.g. sheep), rodents, birds (e.g. chicken) [42]. The role of wildlife is another exogenous factor contributing to the environmental contamination. In fact, movements of wildlife to sub-urban and urban environments due to destruction or reduction of their habitat is another source of soil contamination by T. canis. The key example is represented by synantropic fox populations, which reinforce environmental contamination and risk of infection for humans and stray and domestic dogs [43]. Thus, a combination of these factors is the basis for an extremely high environmental contamination and a life-long risk of infection for dogs living in contaminated areas [44]. Once expelled, T. canis eggs require 2–6 weeks at temperatures of 10–30 C before the eggs are fully embryonated and contain infective L3s. Increasing temperature accelerates the development as well as the degradation of T. canis eggs while temperatures colder than 10 C or warmer (>37 C) are inimical to the maturation or survival of eggs [45]. Embryonation is therefore seasonal in temperate climates but year-round in tropical areas. Eggs are very resistant and survive well over most winters in temperate climates, surviving for 6–12months. Some eggs may be able to survive in moist, cool conditions for 2–4 years or longer [45]. Soil type, pH and percentage of vegetation cover impact upon contamination and viability with clay soils negatively impacting on egg viability [46]. The substrate, light, temperature, humidity, pH and vegetation cover therefore, are important factors for egg availability, development and survival.
Eggs can be physically dispersed by movements of definitive hosts, rainfall, birds, beetles, earthworms, slugs and flies. Studies have concentrated on assessing egg contamination of playgrounds, parks, sandpits, and backyards/gardens and have shown contamination to be prevalent [47]. Although such areas seem to provide opportunities for infection, the role of different domestic and wild definitive hosts in the contamination of these areas is unclear (McPherson, 2013).
Epidemiology Of Human Toxocariasis: Prevalence And Proclivity
Geographical Distribution
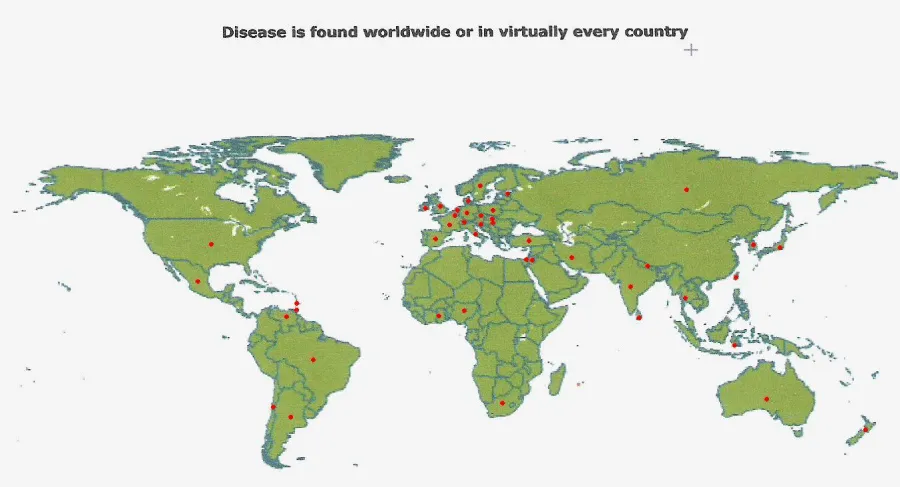
Toxocariasis is found worldwide, although the majority of cases occur where dogs and cats are kept in close proximity to humans (usually household pets). Most cases are reported from the Southeastern United States, Mexico, Hawaii, East and Western Europe, Australia, the Philippines, and South Africa (Fan et al., 2013) (Figure 6).
Within these countries, pet owners (who live in close proximity to infected animals) and children (who are more likely to play in or eat contaminated dirt) are most susceptible to toxocariasis.
Geographical seroprevalence of human infection
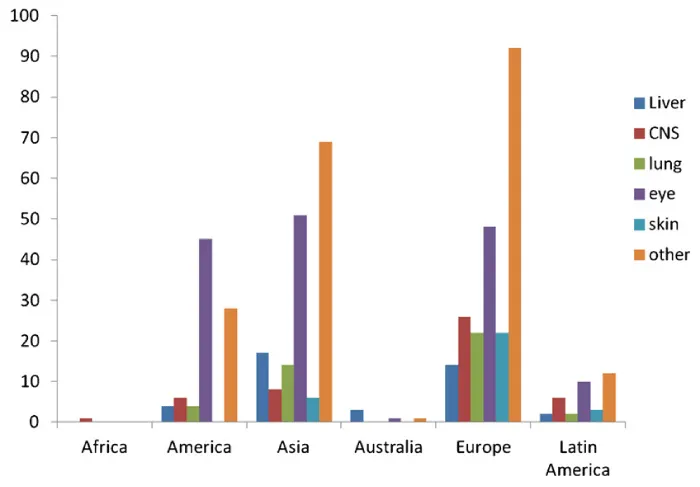
Globally, toxocariasis is found in many countries of the world. Seroprevalence is higher in developing countries, but can be considerable in first world countries, as well. In Bali, St. Lucia, Nepal and other countries, seroprevalence is over fifty percent [9]. Previous to 2007, the U.S. seroprevalence was thought to be around 5% in children [4]. However, Won et al. discovered that U.S. seroprevalence is actually 14% for the population at large [1,48]. In many countries, toxocariasis is considered very rare. Approximately 10,000 clinical cases are seen a year in the U.S., with ten percent being OLM [49]. Permanent vision loss occurs in 700 of these cases (Figure 7).
Seroprevalence of Toxocara in Nigeria: Recent seroprevalence studies conducted in Nigeria have revealed a relatively high seroprevalence of Toxocara in different parts of the country; Ajayi et al. [50], obtained 29.8% seroprevalence in Jos, Plateau state. 86.1% seroprevalence was found among children in Southern Nigeria (Pam et al., 2015). Seroprevalence tests have indicated that approximately 5% of children, and 50% of children who have regular contact with puppies and soil, or who have chronic respiratory problems, carry Toxocara antibodies (Pam et al., 2015).
The global prevalence of Toxocara spp. in humans is influenced by a wide and complex number of variables which are linked at a population level to environmental, geographic, cultural and socioeconomic factors and at the individual level by the heterogeneity of susceptibility to infection influenced by immunity, co-infection, genetics, age, gender, nutrition and behavior of the (human and definitive) hosts Macpherson, 2013 [51]. These factors, together with the increasing human population, global migration and rural: urban migration with more than 50% of the global population now residing in urban areas and the ever closer and denser human-definitive host interactions suggest that, for the majority of the world’s population, Toxocara/ toxocariasis is an ever changing public health problem McPherson, 2013.Transmission and risk factors will vary considerably in different parts of the world. Poverty, a lack of education and problems with uncontrolled and untreated definitive host populations will lead to heavily contaminated environments which, under warm climatic conditions, will provide ideal transmission opportunities, particularly if coupled with poor hygiene and geophagia or pica. These factors, coupled with the ubiquitous distribution of definitive hosts, have resulted in Toxocara spp. being one of the world’s most widespread and prevalent parasitic zoonoses. Such risk factors are widely appreciated and documented for many parts of the world [52].
Risk Factors
There are several factors that have been associated with higher rates of infection with Toxocara. People are more likely to be infected with Toxocara if they own a dog [53]. Children and adolescents under the age of 20 are more likely to test positive for Toxocara infection [54]. Children, due to their behavior and their close contact with dogs, playing in outdoor environment, such as sandboxes where dog and cat faeces can be found, eating soil (pica) [55,56], putting objects in their mouths, and/or their poor hygiene, are most at risk of toxocariasis [1]. Geographic location plays a role as well, because Toxocara is more prevalent in hot, humid regions where eggs are kept viable in the soil [6]. Poverty, a lack of education and problems with uncontrolled and untreated definitive host populations will lead to heavily contaminated environments which, under warm climatic conditions, will provide ideal transmission opportunities [1,52,57,25]. In many cities in Europe, public environments including parks and playgrounds pose as the main area for risk of human exposure to eggs [58]. Embryonated Toxocara spp. eggs have been recovered from the hair of dogs, which demonstrates that direct human-dog contact could also be a route of infection for humans [59,60]. Consumption of raw or undercooked infected viscera or meat has been incriminated [32].
Mode of Infection
Humans become infected by ingesting either embryonated Toxocara eggs from soil [44]; or Toxocara larvae from undercooked giblets (mainly liver) [5]. Humans may also become infected through the ingestion of encapsulated larvae in the raw or undercooked tissues of paratenic hosts such as cows, ostrich, chickens and pigs [61,62], or through unwashed contaminated fruit and vegetables [63]. A new mode of transmission recently proposed is contact with embryonated eggs on a dog’s hair coat [59,60].
Incubation Period
In children, the incubation period can be weeks or months depending on the intensity of the infection and sensitivity of the patient [64]. Ocular manifestations may occur 4 to 10 years after the initial infection. In infections caused by the consumption of infected raw liver, very short incubation periods have been reported.
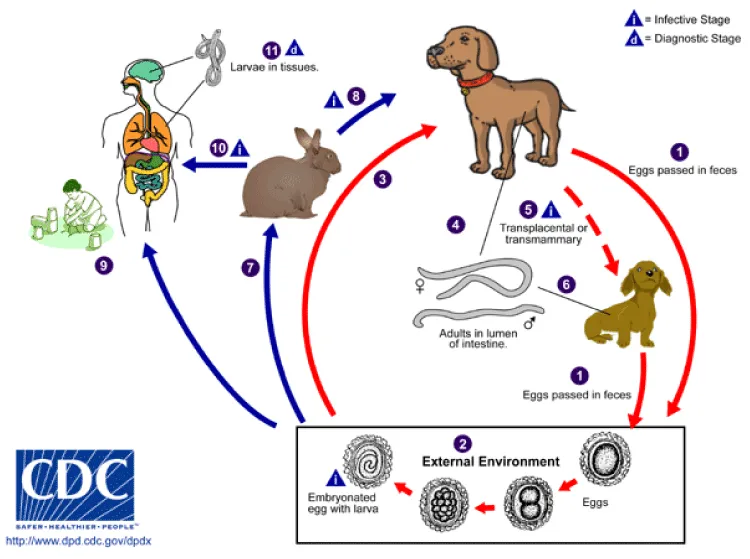
Life Cycle of Toxocara spp (Figure 8)
Toxocara canis accomplishes its life cycle in dogs, with humans acquiring the infection as accidental hosts. Unembryonated eggs are shed in the feces of the definitive host. Eggs embryonate and become infective in the environment. Following ingestion by dogs, the infective eggs hatch and larvae penetrate the gut wall. In younger dogs, the larvae migrate through the lungs, bronchial tree, and esophagus; adult worms develop and oviposit in the small intestine. In older dogs, patent infections can also occur, but larval encystment in tissues is more common. Encysted stages are reactivated in female dogs during late pregnancy and infect by the transplacental and transmammary routes the puppies, in whose small intestine adult worms become established. Puppies are a major source of environmental egg contamination. Toxocara canis can also be transmitted through ingestion of paratenic hosts: eggs ingested by small mammals (e.g. rabbits) hatch and larvae penetrate the gut wall and migrate into various tissues where they encyst. The life cycle is completed when dogs eat these hosts and the larvae develop into egg-laying adult worms in the small intestine. Humans are accidental hosts who become infected by ingesting infective eggs in contaminated soil or infected paratenic hosts. After ingestion, the eggs hatch and larvae penetrate the intestinal wall and are carried by the circulation to a wide variety of tissues (liver, heart, lungs, brain, muscle, eyes). While the larvae do not undergo any further development in these sites, they can cause severe local reactions that are the basis of toxocariasis. The two main clinical presentations of toxocariasis are visceral larva migrans and ocular larva migrans. Diagnosis is usually made by serology or the finding of larvae in biopsy or autopsy specimens.
Clinical Features, Pathology, Diagnosis And Management Of Human Toxocariasis
Clinical Signs, Symptoms and Pathology
Symptoms of toxocariasis vary depending on the affected organ, the magnitude of infection and the intensity of the host inflammatory response Pawlowski, 2001 [7]. The broad spectrum of clinical manifestations in toxocariasis varies from asymptomatic to non-specific clinical signs which make it difficult to directly identify clinical cases of toxocariasis. Therefore, patient clinical history regarding risk factors for Toxocara spp. infection such as occupation, residence, travel history, contact with soil, pets and consumption of raw vegetables or undercooked meats should be gathered as additional information for the diagnosis of toxocariasis [15]. The clinical picture of toxocariasis in humans has been systematically classified in four groups: Visceral larva migrans syndrome (VLM), neurological toxocariasis (NT), ocular larva migrans syndrome (OLM) and the more recently described covert toxocariasis [19]. The severity and range of symptoms depends on the tissue invaded, the number of migrating larvae, and the age of the host.
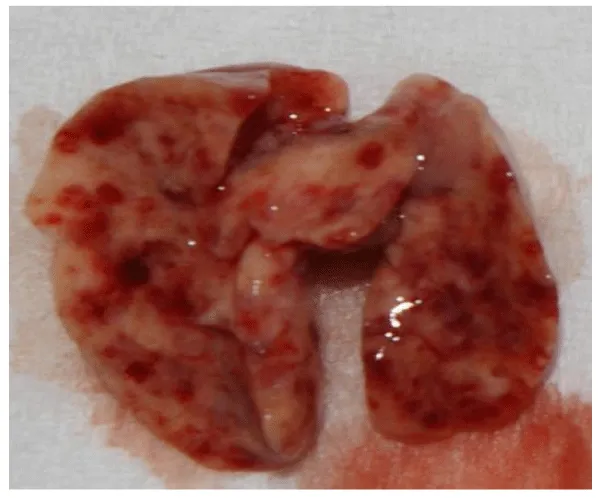
Visceral larva migrans (VLM): The immediate hypersensitivity response to the death of larvae is thought to be the main cause for symptoms of VLM [7]. The first VLM report described a multi-systemic disease with hypereosinophilia and hepatomegaly in three children [16]. Generally young children (<5 years) are most often affected and usually present with fever, abdominal pain, probably due to hepato- and splenomegaly, as well as lower respiratory symptoms such as coughing, bronchospasms and asthma caused by parasitic pneumonia or bronchitis [7,32]. Laboratory diagnosis in these patients commonly reveals leucocytosis, persistent eosinophilia as well as hypergammaglobulinaemia. Other organ involvement such as myocarditis, myalgia with eosinophilic polymyositis, arthritis, and nephritis may also occur. VLM has additionally been associated with dermatological changes such as rash, pruritus, excema, panniculitis, urticara and vasculitis [9]. Although generally most T. canis infections remain unapparent, long-term effects such as development of asthma and promotion of pulmonary fibrosis are suspected to occur [32] (Figure 9).
Neurotoxocariasis: The number of reported cases of neurotoxocarosis is scarce [65]. In contrast, various animal experiments have indicated frequent CNS involvement in paratenic hosts. Migration of Toxocara spp. larvae to the human brain is most often not associated with clinical central nervous signs, but may in rare cases result in eosinophilic meningitis, encephalitis, myelitis or combined pathological presentations [65,66]. Cerebral lesions are predominantly located in the white matter and additional occlusion of cerebral arterial vessels has been reported. Clinical patients present with a large variety of symptoms according to their individual pathology ranging from headache, fever, photophobia, weakness, dorsalgia, confusion, tiredness, visual impairment to epileptic seizures, neuropsychological disturbances, dementia and depression [67]. Furthermore, motor impairment such as ataxia, rigor, para- or tetraparesis and dysaesthesia as well as urinary retention and faecal incontinence occurred in human cases of toxocarosis [65].
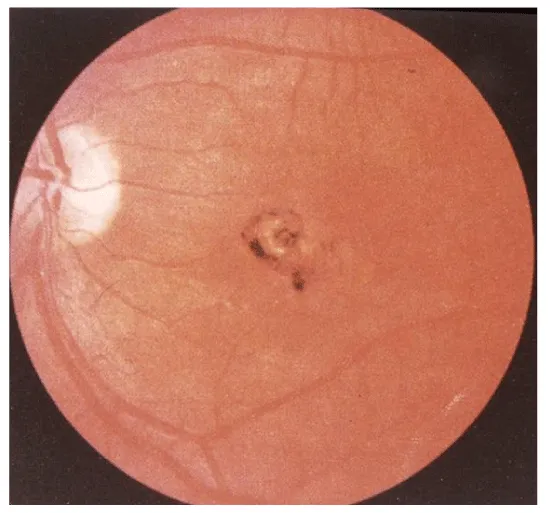
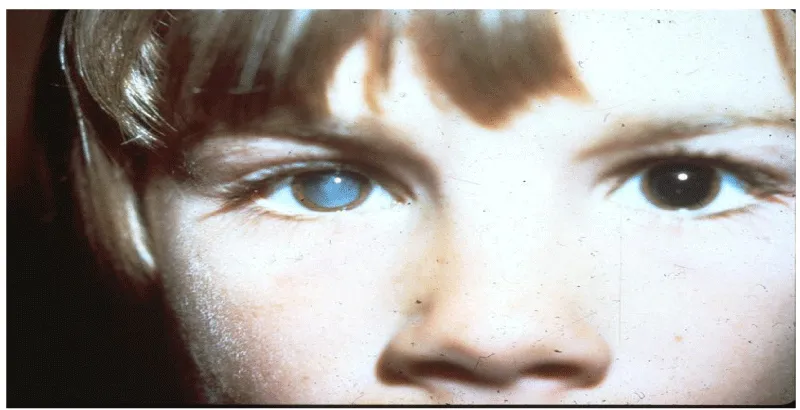
Ocular larva migrans (OLM): Ocular larva migrans syndrome (OLM) is characterized by an eosinophilic immune response to larval migration into the eye. After formation of an eosinophilic abscess, a granulomatous inflammatory reaction surrounds the larvae [68]. Histopathological examinations revealed multiple retinal and vitreous haemorrhages, eosinophilic abscesses and granulomatous lesions with or without larvae. The lack of larvae in some lesions was attributed to destruction of the causative organism and its mobility [68]. OLM occurs predominantly unilaterally. Bilateral ocular involvement has been described but can be considered uncommon [68]. Clinical findings predominantly comprise visual impairment, strabismus, leukocoria, solid retinal mass predominantly at the posterior pole, vitreous mass or haze, vitritis, retinal detachment, cataract, endophthalmitis, papillitis and uveitis. Possible clinical consequences of OLM are blindness and secondary glaucoma [7] (Figures 10-12).
Covert toxocariasis: The term covert toxocarosis was first introduced by Taylor et al. [69], describing a non-specific clinical syndrome in children caused by Toxocara spp. infection which could not be subsumed under VLM, OLM or NT. Unspecific symptoms such as fever, anorexia, nausea, headache, abdominal pain, vomiting, sleep and behaviour disorders, pharyngitis, pneumonia, cough, wheeze, limb pains, cervical lymphadenitis could be observed [69,19].
Other clinical manifestations associated with toxocariasis: Toxocara canis infection has also been associated with asthma and may be linked to the rise in asthma observed in inner city children in cities in the USA [70], and other countries (Kanobana et al., 2013). There is also growing evidence that implicates Toxocara spp. infection in epilepsy (Quattrocchi et al., 2012).
Toxocara and the intestinal flora: The parasite when in contact with other microorganisms in the intestinal flora can result in catabolic wastages. Catabolic wastage through activation of the acute phase response, and interference with the host’s acquisition of nutrients by maldigestion, malabsorption, intestinal losses and competition with the parasite burden can impair growth and nutrition of the host [71]. Also, there exists a defensive interaction. The endogenous flora plays an important role in the development of the immunological defense mechanisms of the intestines, as the secretion of IgA, production of epithelial lymphocytes, and the maturation of the MHC molecules [72]. The use of probiotics prevents the intestinal infection in animals. The mechanism of action of probiotics include competition for intestinal surface receptors, stimulation of humoral immunity, secretion of extracellular factors with antimicrobial activities and competition for intraluminal nutrients [73].
Diagnosis of Human Toxocariasis
Diagnosis and treatment of patients with toxocariasis have been associated with difficulty [5]. Direct microscopic diagnosis of Toxocara spp. larval infection in humans is difficult and rarely attempted. However, methods employed in diagnosis of Toxocariasis include:
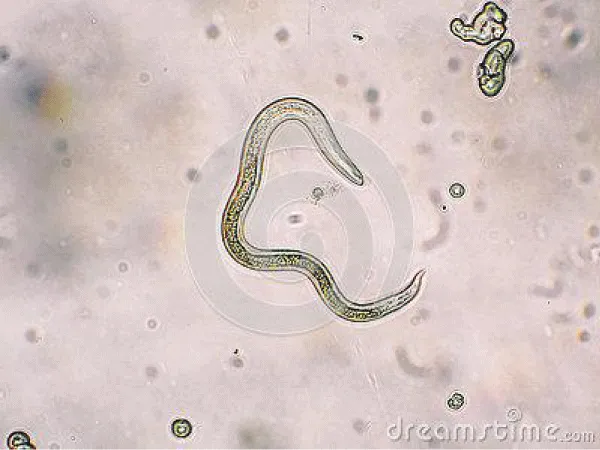
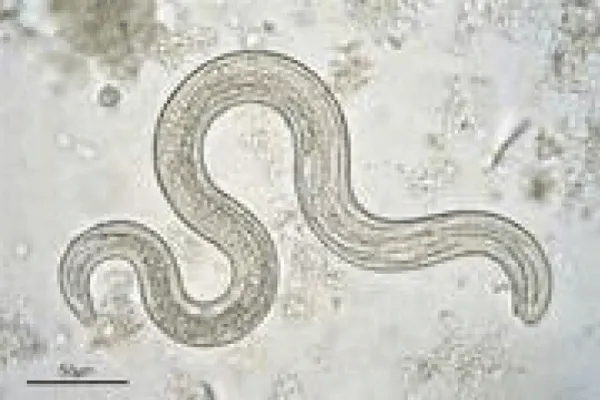
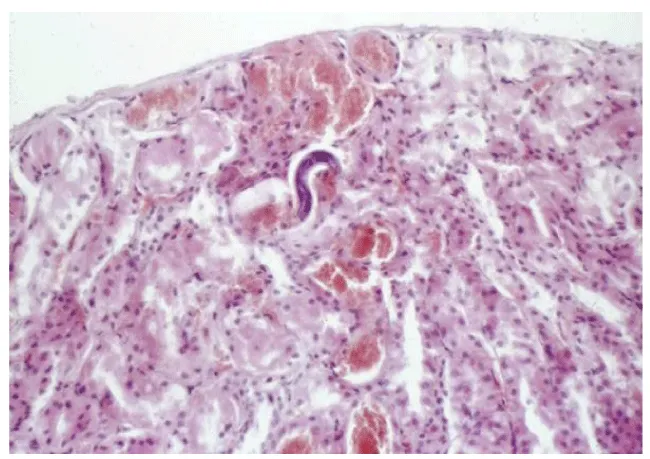
Direct optical diagnosis: A diagnosis of toxocariasis is only confirmed by visual methods (“gold standard”). Gross examination can identify a larva found, e.g., in the CSF or in ocular fluids. Live larvae are highly motile in liquid media. Microscopic examination of biopsies or organ fragments can discover larval sections or debris [74]. T. canis larvae, if undamaged, display the morphological characteristics of larval nematodes. On average, they are 400 μm in length and 18–20 μm in breadth. The edges are parallel to the bulk of the body, so the larvae appear squat. The length of the esophagus is about one-third of the total body length [75]. On pathological examinations, the cuticle presents two characteristic lateral alae [76]. T. cati larvae have a similar morphology with T. canis [75]. The morphological identification of Toxocara infection by species eggs has been documented to give minor differences. Scanning electron microscopic observation was able to differentiate T. canis eggs from T. cati eggs based on their respective characteristic surface structures. Both species have subspherical eggs with marked pitted surfaces, but the surface pitting of T. canis is coarser than that in T. cati [77].
Immunodiagnosis: Development of serological methods was prompted by the difficulties and uncertainties of direct visual diagnosis [74]. To confirm suspected toxocariasis, it is recommended that patients should always be examined with serodiagnostic tests using at least two consecutive serum samples taken approximately 2 weeks apart [15]. An ELISA detecting IgG antibodies against Toxocara Excretory-Secretory (TES) Ag (IgG TES-ELISA) has become the reference test for toxocariasis immunodiagnosis. For the serodiagnosis of the generalized forms of toxocariasis, VLM or common/ covert toxocariasis, the best choice relies upon the initial use of Toxocara Excretory-Secretory antigen in Enzyme Linked Immunosorbent Assay (TES-ELISA), after which any positive result should be subsequently tested by western blotting (WB). There is great interest in this combined diagnostic procedure because TES-ELISA provides fast and relatively inexpensive negative results. The only current concern is the diagnostic value of a positive result, which is constantly obscured by the presence of a more or less elevated seroprevalence rate. Common/ covert toxocariasis is mostly a benign infection, so a large majority of infected subjects are asymptomatic or have very few symptoms and go undiagnosed. In this form, this helminthiasis is often self-limiting and leaves residual specific antibodies. On average, the duration of a positive TES-ELISA result would be 2.7 years, and over 5 years for WB [19]. A positive serodiagnostic caused by residual antibodies that do not have any diagnostic significance can therefore be associated with any infectious or non-infectious disease. If separated from the ongoing clinical and laboratory context, such a positive result has no diagnostic value and should be only taken into account after the possible etiologies of the observed syndromes – including blood eosinophilias– have been ruled out [74].
Immunodiagnosis of ocular and neurological compartmentalized forms represents a difficult problem. The parasite burden is always tiny and often reduced to one larva; thus an immunodiagnostic test carried out on serum usually is negative [78]. For ocular toxocariasis, immunodiagnosis is made possible using Aqueous Humor (AH) in a TES-ELISA [79] or WB [80]. Both methods detect specific IgG. For neurological toxocariasis, immunodiagnosis can be carried out on CSF [78]. Immunodiagnosis on AH or CSF should be always coupled with serum testing. If the results are discordant namely negative on serum but positive AH or CSF, this finding indicates intraocular or intrathecal synthesis of specific anti-ES Ag IgG. When serum and any aspirated fluid are simultaneously positive, synthesis of specific IgG in the eye or CNS should be assessed using Reiber’s formula [81].
Treatment
Albendazole is the treatment of choice for toxocariasis. Patients receiving a 5-day treatment course of albendazole (10 mg/kg of body weight/day in two divided doses) improved relative to patients who received treatment with the older anthelminthic drug, thiabendazole. A dose of 400 mg of albendazole twice a day for 5 days is the currently recommended therapy. Because the other commonly used benzimidazole, mebendazole, is poorly absorbed outside the gastrointestinal tract, this agent is a second-line treatment, although some success has been reported in patients who ingest 1 g or more for a 21-day course. Symptomatic treatment, including administration of corticosteroids, has been helpful for suppressing the intense allergic manifestations of the infection [7].
For ocular toxocariasis, the goal of treatment is to minimize damage to the eye. Systemic antiparasitic treatment with albendazole or mebendazole at the same doses as for visceral disease may be beneficial for active disease. Attempts to surgically remove the larva may be unsuccessful. Control of inflammation in the eye by use of topical or systemic steroids may be indicated. For patients with quiescent disease, improved outcomes may result from surgical intervention to prevent further damage due to chronic inflammation.
For the eradication of eggs in the soil, it had been reported to be impractical [15]. However, the following soil treatment procedures worthy of note include rigorous faecal removal practices, regularly replacing sand or sterilizing it, and eliminating sandpits or sandcastles altogether in public areas [82].
Prevention and Control
Pet owners need to involve veterinarians in controlling the transmission of Toxocara from pets to humans. Since pregnant or lactating dogs and cats and their offspring have the highest, active parasitic load, these animals should be placed on a deworming program (Holland et al., 2006; CDC, 2014). Regular anthelmintic treatment, particularly in puppies and kittens, will reduce the number of infectious eggs in the environment [25].
Reduce contact with contaminated soil. When working with soil (through gardening or other activities), it is important to wear gloves [56]. Pet faeces should be picked up and disposed of or buried, as they may contain Toxocara eggs (CDC, 2009). Practicing this measure in public areas, such as parks and beaches, is especially essential for decreasing transmission [7]. Also, sandboxes should be covered when not in use to prevent cats from using them as litter boxes. Hand washing after working on contaminated soil [15], before eating and after playing with pets, as well as after handling dirt will reduce the chances of ingesting Toxocara eggs.
Washing all fruits and vegetables [9,63], keeping pets out of gardens and thoroughly cooking meats can also prevent transmission. Finally, teaching children not to place non-food items, especially dirt, in their mouths will drastically reduce the chances of infection. Toxocara spp. eggs are very resistant to adverse environmental conditions and remain infective for years. Since no practical methods exist for reducing environmental egg burdens, prevention of initial contamination of the environment is the most important tool [15]. In order to increase awareness of the potential zoonotic hazards, veterinary practitioners, general practitioners and public health agencies should provide sufficient information and advice for minimizing the risk of infection. Health education and discouraging geophagia in children are fundamental [82]. Continuous education with emphasis on zoonotic risks is strongly recommended.
Conclusion
Toxocariasis remains a problem throughout the world, causing multisystem disease, especially in young people. Prevalence of parasite infection in dogs with importance for human health is usually high, resulting in risk of zoonotic transmission from dogs to humans [83-96]. Co-habitation of human with dog and cat pets enhances transmission of Toxocara. Contact with infected dog, especially pups, is a risk factor for infection, which is a reason for concern in term of public health because the presence of dogs in urban areas is becoming increasingly frequent. Researches on the prevalence of toxocariasis in Nigeria are limited and the high seroprevalence revealed in recent studies is of great public health importance. Several individuals are exposed to various risks associated with toxocariasis while some apparently healthy individual could be infected with Toxocara and are at risk of developing visceral toxocariasis, covert toxocariasis, or ocular toxocariasis and associated complications. There is need for public awareness campaign on toxocariasis and associated risks as well as further researches to ascertain seroprevalence in areas not yet covered. Mass therapy of infected individuals is important to reduce morbidity and permanent disabilities from toxocariasis. Pet owners need to be educated on regular pet care and pet treatment with benzimidazoles to reduce worm burden. Toxocariasis and associated risks can best be curtailed by preventive measures such as personal and environmental hygiene. Advances in the development of serological methodology and controlled studies that can define the acute and chronic stages of the disease are needed for the follow-up of patients and control of the infection cure.
- (2013)Centre for Disease Control and Prevention. Parasites: toxocariasis (also known as roundworm infection). Assessed on March 15, 2017. Link: https://goo.gl/QkzceV
- Momeni T, Mahami-Oskouei M, Fallah E, Safaiyan A, Mahami-Oskouei L (2016) Latent and asymptomatic Toxocara infection among young population in northwest Iran: the necessity of informing people as a potential health risk. Scientifica 2016: 3562056. Link: https://goo.gl/Jd48mA
- Meyers WM, Neafie RC, Marty AM, Wear DJ (2000) Helminthiases. In: Meyers WM, Neafie RC, Marty AM, Wear DJ (Editors). Pathology of Infectious Diseases, Volume I. Armed Forces Institute of Pathology, Washington, DC. Link: https://goo.gl/sDHeF5
- Markell EK, Marietta V (2006) Markell and Voge’s Medical Parasitology, Nineth Edition. Saunders Elsevier, St. Louis. Link: https://goo.gl/QxHcv3
- Moreira GM, TelmoPde L, Mendonça M, Moreira AN, McBride AJ, et al. (2014) Human toxocariasis: current advances in diagnostics, treatment, and interventions. Trends Parasitol 30: 456-464. Link: https://goo.gl/TZV4Zg
- McGuiness SL, Leder K (2014) Global burden of Toxocariasis: a common neglected infection of poverty. Current Tropical Medicine Reports 1: 52-61. Link: https://goo.gl/kxWmTR
- Despommier D (2003) Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev 16: 265-272. Link: https://goo.gl/BV217m
- Oryan A, Alidadi S (2015) Toxocarasis: a neglected parasitic diseases with public health importance. Tropical Medicine and Surgery 3: e126. Link: https://goo.gl/dYFWZ8
- Holland CV, Smith HV (2006) Toxocara: The Enigmatic Parasite. CABI Publishing, Wallingford, UK. Link: https://goo.gl/cUATdV
- Fan CK, Holland CV, Loxton K, Barghouth U (2015) Cerebral toxocariasis: silent progression to neurodegenerative disorders. Clin Microbiol Rev 28: 663 - 686. Link: https://goo.gl/2SMH1H
- Moorhouse DE (1982) Toxocariasis: a possible cause of the Palm Island mystery disease. The Medical Journal of Australia 1: 172-173. Link: https://goo.gl/hYbLVi
- Gibbons LM1, Jacobs DE, Sani RA (2001) Toxocara malaysiensis (Nematoda: Ascaridoidea) from domestic cat (Felis catus Linnaeus 1758). Journal of Parasitology 87: 660-665. Link: https://goo.gl/2RtufD
- Macchioni G (1999) A new species Toxocara lyncis, in the caracal (Lynx caracal). Parasitology 41: 529-532. Link: https://goo.gl/KXUSnh
- Iddawela DR, Kumarasiri PV, de Wijesundera MS (2003) A seroepidemiological study of toxocariasis and risk factors for infection in children in Sri Lanka. Southeast Asian Journal of Tropical Medicine and Public Health 34: 7-15. Link: https://goo.gl/aK9onq
- Hamilton CM, Yoshida A, Pinelli E, Holland CV, Bruschi F (2014) Toxocariasis. In: Hamilton CM, Yoshida A, Pinelli E, Holland CV, Bruschi F (Editors). Helminth Infections and their Impact on Global Public Health 425-460. Link: https://goo.gl/dbpbHi
- Beaver PC, Snyder CH, Carrera GM, Dent JH, Lafferty JW (1952) Chronic eosinophilia due to visceral larva migrans; report of three cases. Pediatrics 9: 7 – 19. Link: https://goo.gl/6eE9SU
- Xi W, Jin L (1998) A novel method for the recovery of Toxocara canis in mice. J Helminthol72: 183-184. Link: https://goo.gl/4U7oie
- Helwigh A, Lind P, Nansen P (1999) Visceral larva migrans: migratory pattern of Toxocara canis in pigs. International Journal of Parasitology 29: 559 – 565. Link: https://goo.gl/Wr7MZs
- Magnaval JF, Glickman LT, Dorchies P, Morassin B (2001) Highlight of human toxocariasis. Korean J Parasitol 39: 1-11. Link: https://goo.gl/wESCRx
- Pinelli E, Kortbeek LM, van der Giessen JWB (2006) Toxocara. In: Cox FEG, Wakelin D, Gillespie SH, Despommier DD (Editors). Topley & Wilson's Microbiology and Microbial Infections. Tenth Edition. Wiley-Blackwell, Oxford, UK.
- Harris-Linton M (2001) Toxocara canis (On-line), Animal Diversity Web. Assessed August 05, 2016. Link: https://goo.gl/iE4mZ8
- Brunaska M, Dubinsky P, Teiterova K (1995) Toxocara canis: ultrasturctual aspects of larval moulting in the maturing eggs. Int J Parasitol 25: 683-690. Link: https://goo.gl/n2M5tj
- Roberts L, Janvory J (2000) Gerald. D. Schmidt & Larry S. Roberts' Foundations of Parasitology. Sixth Edition. McGraw-Hill Companies, Inc., United States.
- Barnes R (1987) Invertebrate Zoology. Dryden Press, Orlando, Florida. Link: https://goo.gl/Jn88hZ
- Overgaauw PA, van Knapen F (2013) Veterinary and public health aspects of Toxocara spp. Veterinary Parasitology193: 398-403. Link: https://goo.gl/Etn56F
- Lloyd S (1993) Toxocara canis: the dog. In: Lewis JW, Maizels RM (Editors). Toxocara and Toxocariasis: Clinical, Epidemiological and Molecular Perspectives. Institute of Biology, London.
- Morgan ER, Azam D, Pegler K (2013) Quantifying sources of environmental contamination with Toxocara species eggs. Veterinary Parasitology 193: 390-397. Link: https://goo.gl/Yyffqy
- Claerebout E, Casaert S, Dalemans AC, De Wilde N, Levecke B, et al. (2009) Giardia and other intestinal parasites in different dog populations in northern Belgium. Veterinary Parasitology 161: 41-46. Link: https://goo.gl/3ruYNS
- Okoye IC, Obiezue NR, Okorie CE, Ofoezie IE (2011) Epidemiology of intestinal helminth parasites in stray dogs from markets in south-eastern Nigeria. J Helminthol 85: 415 – 420. Link: https://goo.gl/2Q3TJ1
- Lloyd S (2006) Seroprevalence of Toxocara canis in sheep in Wales. Vet Parasitol 137: 269-272. Link: https://goo.gl/Gc3Zsp
- Choi D, Lim JH, Choi DC, Lee KS, Paik SW, et al. (2012) Transmission of Toxocara canis via ingestion of raw cow liver: a cross-sectional study in healthy adults. Korean J Parasitol 50: 23-27. Link: https://goo.gl/EvzATv
- Strube C, Heuer L, Janecek E (2013) Toxocara spp infections in paratenic hosts. Veterinary Parasitology 193: 375-389. Link: https://goo.gl/SpKjyA
- Cox DM, Holland CV (2001) Relationship between three intensity levels of Toxocara canis larvae in the brain and effects on exploration, anxiety, learning and memory in the murine host. J Helminthol 75: 33-41. Link: https://goo.gl/e6Xw4K
- Santos SV, Lescano SZ, Castro JM, Chieffi PP (2009) Larval recovery of Toxocara cati in experimentally infected Rattus norvegicus and analysis of the rat as potential reservoir for this ascarid. Mem Inst Oswaldo Cruz 104: 933-934. Link: https://goo.gl/7UJD8o
- Overgaauw PA (1997) Aspects of Toxocara epidemiology: toxocariasis in dogs and cats. Crit Rev Microbiol 23: 233-251. Link: https://goo.gl/22bUFG
- Moore TA, McCarthy JS (2006) Toxocariasis and larva migrans syndromes. In: Guerrant RL, Walker DH, Weller PF (Editors). Tropical Infectious Diseases: Principles, Pathogens and Practice. Elsevier Churchill Livingstone, Philadelphia, PA.
- Emehelu CO, Fakae BB (1986) Prevalence of Toxocara canis ova on playgrounds of nursery schools in Nsukka, Nigeria. Int J Zoonoses 13: 158-161. Link: https://goo.gl/k4FFdV
- Umeche N (1989) Helminth ova in soil from children’s playgrounds in Calabar. Cent Afr J Med 35: 432-434. Link: https://goo.gl/5p2LEC
- Maikai BV, Umoh JU, Ajanusi OJ, Ajogi I (2008) Public health implications of soil contaminated with helminth eggs in the metropolis of Kaduna, Nigeria. J Helminthol 82: 113-118. Link: https://goo.gl/5HAQ8r
- Kirchheimer R, Jacobs DE (2008) Toxocara species egg contamination of soil from children’s play areas in southern England. Vet Rec 163: 394-395. Link: https://goo.gl/TeP9Uu
- Mizgajska-Wiktor H, Uga S (2006) Exposure and environmental contamination. In: Holland CV, Smith H (Editors). Toxocara: The Enigmatic Parasite. CABI Publishing, Wallingford, UK. Link: https://goo.gl/Pg2Vye
- Traversa D (2012) Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors 5: 91-100. Link: https://goo.gl/ueWLiL
- Brochier B, De Blander H, Hanosset R, Berkvens D, Losson B, et al. (2007) Echinococcus multilocularis and Toxocara canis in urban red foxes (Vulpes vulpes) in Brussels, Belgium. Prev Vet Med 80: 65-73. Link: https://goo.gl/MTdJGs
- Donato T, Anthonio F, Angela C, Francesco T, Jason D, et al. (2014) Environmental contamination by canine geohelminths. Parasit Vectors 7: 67. Link: https://goo.gl/eQMq3Y
- Azam D, Ukpai OM, Said A, Abd-Allah GA, Morgan ER (2012) Temperature and the development and survival of infective Toxocara canis larvae. Parasitology Research 110: 649-656. Link: https://goo.gl/ajs6Gp
- Trejo CAC, Nunez CR, Contreras ACG, Barrera GEM (2012) Soil contamination by Toxocara spp. eggs in a University in Mexico City. Rev Bras Parasitol Vet 21: 298-300. Link: https://goo.gl/pWF4y2
- Manini MP, Marchioro AA, Colli CM, Nishi L, Falavigna-Guilherme AL (2012) Association between contamination of public squares and seropositivity for Toxocara spp. in children. Vet Parasitol 188: 48-52. Link: https://goo.gl/aoBR36
- Won KY, Kruszon-Moran D, Schantz PM, Jones JL (2008) National seroprevalence and risk factors for zoonotic Toxocara spp. infection. Am J Trop Med Hyg 79: 552-557. Link: https://goo.gl/nnsc9N
- (2009) Centre for Disease Control and Prevention. New CDC study results show Toxocara infection more common than previously thought. Assessed on March 15, 2017. Link: https://goo.gl/QPR6jc
- Ajayi OO, Duhlinska DD, Agwale SM, Njoku M (2000) Frequency of human toxocariasis in Jos, Plateau State, Nigeria. Mem Inst Oswaldo Cruz 95: 147-149. Link: https://goo.gl/x32qRR
- Dana MW, Mark LE, Monica EP (2014) Neglected parasitic infections in the United States: toxocariasis. Am J Trop Med Hyg 90: 810-813. Link: https://goo.gl/89hcnW
- Lee AC, Schantz PM, Kazacos KR, Montgomery SP, Bowman DD (2010) Epidemiologic and zoonotic aspects of ascarid infections in dogs and cats. Trends Parasitol 26: 155-161. Link: https://goo.gl/vjp9oQ
- Jarosz W, Mizgajska-Wiktor H, Kirwan P, Konarski J, Rychlicki W, et al. (2010) Developmental age, physical fitness and Toxocara seroprevalence amongst lower-secondary students living in rural areas contaminated with Toxocara eggs. Parasitology 137: 53-63. Link: https://goo.gl/MGNKyd
- Fan CK, Hung CC, Du WY, Liao CW, Su KE (2004) Seroepidemiology of Toxocara canis infection among mountain aborigina: school children living in contaminated districts in eastern Taiwan. Trop Med Int Health 9: 1312-1318. Link: https://goo.gl/Vhx766
- Lewis JW (2006) Epidemiological surveillance of Toxocara and Toxocariasis. In: Holland CV, Smith H (Editors). Toxocara: The Enigmatic Parasite. CABI Publishing, Wallingford, UK. Link: https://goo.gl/92Kv4D
- Negri EC, Santarem V, Rubinsky-Elefant G, Giuffrida R (2013) Anti-Toxocara spp. antibodies in an adult healthy population: serosurvey and risk factors in Southeast Brazil. Asian Pac J Trop Biomed 3: 211-216. Link: https://goo.gl/dwa9Kq
- Congdon P, Lloyd P (2011) Toxocara infection in the United States: the relevance of poverty, geography and demography as risk factors, and implications for estimating country prevalence. International Journal of Public Health 56: 15-24. Link: https://goo.gl/RqB2nM
- Deplazes P, van Knapen F, Schweiger A, Overgaauw PA (2011) Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet Parasitol 182: 41-53. Link: https://goo.gl/aTt7Ze
- Roddie G, Stafford P, Holland C, Wolfe A (2008) Contamination of dog hair with eggs of Toxocara canis. Vet Parasitol 152: 85-93. Link: https://goo.gl/BcgjRW
- El-Tras WF, Holt HR, Tayel AA (2011) Risk of Toxocara canis eggs in stray and domestic dog hair in Egypt. Vet Parasitol 178: 319-323. Link: https://goo.gl/ACF8gz
- Yoshikawa M, Nishiofuku M, Moriya K, Ouji Y, Ishizaka S, et al. (2008) A familial case of visceral toxocariasis due to consumption of raw bovine liver. Parasitology International 57: 525-529. Link: https://goo.gl/dXgcgG
- Noh Y, Hong ST, Yun JY, Park HK, Oh JH, et al. (2012) Meningitis by Toxocara canis after ingestion of raw ostrich liver. J Korean Med Sci 27: 1105-1108. Link: https://goo.gl/c6cU2W
- Klapec T, Borecka A (2012) Contamination of vegetables, fruits and soil with geohelminths eggs on organic farms in Poland. Annals of Agricultural and Environmental Medicine 19: 421-425. Link: https://goo.gl/f3Hhwa
- Moreira G, Telmo P, Mendonca M, Moreira AN (2004) In: Heymann DL (Editor). Control of Communicable Diseases Manual. Eighteenth Edition. American Public Health Association, Washington, DC. Link: https://goo.gl/8ULk5W
- Moreira-Silva SF, Rodrigues MG, Pimenta JL, Gomes CP, Freire LH, et al. (2004) Toxocariasis of the central nervous system: with report of two cases. Rev Soc Bras Med Trop37: 169-174. Link: https://goo.gl/wr5W7F
- Caldera F, Burlone ME, Genchi C, Pirisi M, Bartoli E (2013) Toxocara encephalitis presenting with autonomous nervous system involvement. Infection 41: 691-694. Link: https://goo.gl/LHvbdU
- Richartz E, Buchkremer G (2002) Cerebral toxocariasis: a rare cause of cognitive disorders: a contribution to differential dementia diagnosis. Nervenarzt 73: 458-462. Link: https://goo.gl/1J5MKU
- Taylor MR (2006) Ocular toxocariasis. In: Holland CV, Smith HV (Editors). Toxocara: The Enigmatic Parasite. CABI Publishing, Wallingford, UK.
- Taylor MR, Keane CT, O’Connor P, Girdwood RW, Smith H (1987) Clinical features of covert toxocariasis. Scand J Infect Dis 19: 693-696. Link: https://goo.gl/f8yZaF
- Walsh MG, Haseeb MA (2012) Reduced cognitive function in children with toxocariasis in a nationally representative sample of the United States. Int J Parasitol 42: 1159 – 1163. Link: https://goo.gl/Z1U5ny
- Solomons NW (1993) Pathways to the impairment of human nutritional status by gastrointestinal pathogens. Parasitology 107: S19 – S35. Link: https://goo.gl/JhRoY5
- Umesaki Y, Setoyama H (2000) Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microbes Infect 2: 1343-1351. Link: https://goo.gl/pQWGxq
- Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130(2S Suppl): 396S-402S. Link: https://goo.gl/ZXCin5
- Fillaux J, Magnaval F (2013) Laboratory diagnosis of human toxocariasis. Vet Parasitol 193: 327-336. Link: https://goo.gl/Ywgtwc
- Nichols RL (1956) The etiology of visceral larva migrans I: diagnostic morphology of infective second-stage Toxocara larvae. J Parasitol 42: 349-362. Link: https://goo.gl/szCy5p
- Marty AM (2000) Toxocariasis. In: Meyers WM, Neafie RC, Marty AM, Wear DJ (Editors). Pathology of Infectious Diseases, Volume I. Armed Forces Institute of Pathology, Washington, DC.
- Uga S, Matsuo J, Kimura D, Rai SK, Koshino Y, et al. (2000) Differentiation of Toxocara canis and Toxocara cati eggs by light and scanning electron microscopy. Vet Parasitol 92: 287-294. Link: https://goo.gl/CiRNTR
- Vidal JE, Sztajnbok J, Seguro AC (2003) Eosinophilic meningoencephalitis due to Toxocara canis: case report and review of the literature. Am J Trop Med Hyg 69: 341-343. Link: https://goo.gl/n36MKD
- de Visser L, Rothova A, de Boer JH, van Loon AM, Kerkhoff FT, et al. (2008) Diagnosis of ocular toxocariasis by establishing intraocular antibody production. Am J Ophthalmol 145: 369-374. Link: https://goo.gl/scuSJj
- Magnaval JF, Malard L, Morassin B, Fabre R (2002) Immunodiagnosis of ocular toxocariasis using Western-blot for the detection of specific anti-Toxocara IgG and CAP for the measurement of specific anti-Toxocara IgE. J Helminthol 76: 335-339. Link: https://goo.gl/eSkVze
- Reiber H, Felgenhauer K (1987) Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin Chim Acta163: 319-328. Link: https://goo.gl/6N4szu
- Otero D, Alho AM, Nijsse R, Roelfsema J, Overgaauw P, Madeira de Carvalho L (2017) Environmental contamination with Toxocara spp. eggs in public parks and playground sandpits of Greater Liston, Portugal. J Infect Public Health. Link: https://goo.gl/LvPahq
- Ugbomoiko US, Ariza L, Heukelbach J (2008) Parasites of importance for human health in Nigerian dogs: high prevalence and limited knowledge of pet owners. BMC Vet Res 4: 49. Link: https://goo.gl/Ap27bs
- Błaszkowska J, GóralskaK, WójcikA,Kurnatowski P,SzwabeK (2015). Presence of Toxocara spp. eggs in children’s recreation areas. Ann Agric Environ Med 22: 23-37. Link: https://goo.gl/VRDXPw
- (2014) Centre for Disease Control and Prevention. Parasites: toxocariasis (also known as roundworm infection). Assessed on March 15, 2017. Link: https://goo.gl/2Dv8q1
- Chia-Kwung F, Chien-Wei L, Yu-Chieh C (2013) Factors affecting disease manifestation of toxocarosis in humans: genetics and environment. Vet Parasitol 193: 342-352. Link: https://goo.gl/L9zRH1
- Duggan IC, Collier KJ, Lambert PW (2002) Evaluation of invertebrate biometrics and the influence of subsample size using data from some Westland, New Zealand, lowland streams. New Zealand Journal of Marine and Freshwater Research 36: 117-128.
- (2017) GIDEON Database. Assessed on March 15, 2017.
- Gyang PV, Akinwale OP, Lee YL, Chuang TW, Orok AB, et al. (2015) Seroprevalence, disease awareness, and risk factors for Toxocara canis infection among primary school children in Makoko, an urban slum community in Nigeria. Acta Tropica 146: 135-140. Link: https://goo.gl/p8FhFQ
- Hasby K, Senyonjo L, Gupta S, Ladburg G, Suvari M, et al. (2016) Epidemiology of toxocariasis in England and Wales. Zoonoses and Public Health 63: 529-533. Link: https://goo.gl/ZSiDV7
- Jeanfaivre T, Cimon B, Tolstuchow N, de Gentile L, Chabasse D (1996) Pleural effusion and toxocariasis. Thorax 51: 106-107. Link: https://goo.gl/tTbJPj
- Kagan IG (1957) Serum-agar double diffusion studies with Ascaris antigens. J Infect Dis 101: 11-19. Link: https://goo.gl/egdn1V
- Kagan IG (1958) Hemagglutination tests with Ascaris antigens. J Immunol 80: 396-399. Link: https://goo.gl/CN1Eah
- Magnaval JF, Glickman LT (2006) Management and treatment options for human toxocariasis. In: Holland CV, Smith HV (Editors). Toxocara: The Enigmatic Parasite. CABI Publishing, Wallingford, UK. Link: https://goo.gl/WAbDWd
- Takayanagi T, Akao N, Suzuki A, Tomoda M, Tsukidate S (1999) New animal model for human ocular toxocariasis: ophthalmoscopic observation. Br J Ophthalmol 83: 967-972. Link: https://goo.gl/RrnWsB
- Wilder HC (1950) Nematode endophthalmitis. Trans Am Acad Ophthalmol Otolaryngol 55: 99-109. Link: https://goo.gl/VFWQGm
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
