Archive of Gerontology and Geriatrics Research
Development of a new drug for progeria syndrome; Past, Present and Future
So-mi Kang1, Minju Kim2 and Bum-Joon Park1*
2PRG Science & Technology, BUSAN, Korea
Cite this as
Kang SM, Kim M, Park BJ (2020) Development of a new drug for progeria syndrome; Past, Present and Future. Arch Gerontol Geriatr Res 5(1): 022-025. DOI: 10.17352/aggr.000020Hutchinson-Gilford progeria syndrome (HGPS) is the best characterized genetic disorder with premature aging features. Classic HGPS is very rare, sporadic orphan disease, inherited in an autosomal dominant manner without gender or ethnic differences. Children with HGPS appear normal at birth, but begin to develop segmental progeroid symptoms within the first years of life. Patients suffer from sarcopenia, lipodystrophy, diabetes, cataracts, atherosclerosis but not cancer and neurodegenerative diseases. Genetic factors that are associated with this syndrome have been identified, mouse models of disease have been developed, and clinical studies have been conducted for many years. Although many medical and treatment approaches were implemented and showed some efficacy, these therapies could not be considered as a complete cure, and more effective therapeutic approaches were needed for HGPS patients. This report introduces a novel drug called progerinin, which is a binding inhibitor of lamin A and progerin, for patients affected with HGPS.
Introduction
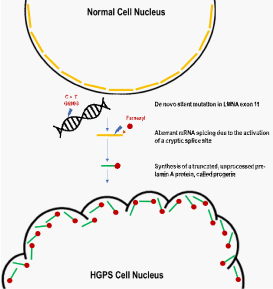
Hutchinson-Gilford progeria syndrome (HGPS) is the most well-known premature aging related diseases [1,2]. Classical HGPS is a rare genetic disorder which affects 1 in 4-8 million new births with aging related features that include thin skin, growth impairment, severe loss of subcutaneous fat, osteoporosis, alopecia, sarcopenia and heart disease leading to short life span and death at about 15 years [3-6]. Most of HGPS cases are caused by substitution of a single base pair at exon 11 of the LMNA gene (c.1824C>T, p.G608G) that encodes lamin A and C [2,7]. Normally lamin A is synthesized as a precursor called pre-lamin A. Pre-lamin A undergoes cysteine farnesylation by farnesyl transferase (FTase) on its C-terminal CaaX motif, and then first cleavage of the amino acids by the metallopeptidase ZMPSTE24. Second cleavage of the amino acids by ZEMPSTE24 is processed after carboxymethylating the C-terminal cysteine by the isoprenylcystein carboxylmethyltransferase (ICMT) to produce the unfarnesylated, mature lamin A [8]. The mutation in HGPS patients activates a cryptic donor splice site in the LMNA gene and leads to deletion of 50 amino acids near the C terminus, abrogating the second cleavage site which is needed for maturation of prelamin A and resulting in accumulation of a truncated and permanently farnesylated prelamin A called progerin (Figure 1). Progerin incorporates abnormally into the nuclear membrane exerting multiple toxic effects [9]. Based on this, the inhibitor of farnesyltransferase (lonafarnib) has been used as a clinical trial for patients with HGPS [10]. Although it has shown some efficacy in HGPS, this drug has revealed side effects and has not worked in all patients [11]. Moreover, farnesylation-defective progerin still evokes nuclear membrane abnormality and promotes cellular senescence (our unpublished data). Thus, inhibition of FTI seems to be not proper strategy for HGPS. In this review, we provide the overview of process of new drug development, based on the new molecular mechanism (inhibition of the interaction of progerin and lamin A/C).
Direct interaction between lamin A/C and progerin
Several reports dealing with proteomic studies and 2-hybrid approaches have identified lamin A/C as a progerin-binding protein [12,13]. Knowledge of these studies presupposed that interaction of progerin with lamin A/C would contribute to the development of the senescence features of HGPS. In 2016, we reported that C-terminal region of progerin strongly interact with lamin A and C but not lamin B1 [14]. The above study indicated that nuclear lamina alteration can be derived from the strong binding of progerin to lamin A and C and predicted that progerin/lamin A-binding inhibitors would be effective in HGPS.
Specific binding inhibitors of progerin and lamin A
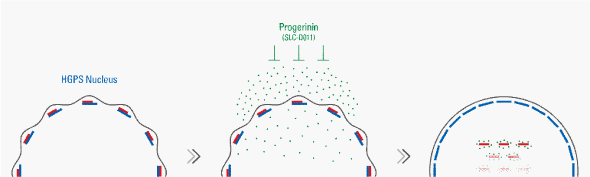
Based on our hypothesis, we screened the chemical library and found that JH4 chemical can block the interaction of progerin and lamin A. Indeed, JH4 bound to progerin directly and block the interaction of progerin and lamin A. In addition, JH4 ameliorated nuclear deformation, decreased progerin expression, and rescued the chromatin phenotype of cultured HGPS fibroblasts. Studies on LmnaG609G mouse model for the human HGPS, showed that pharmacologic inhibition of progerin interaction with lamin A by JH4 improves body weight and skeletal muscle function and enhances survival rate [14]. Although therapeutic effect of JH4 was favorable, it showed extremely short half-life in in vivo oral administration. To improve its in vivo stability, various JH4 modified chemicals were generated. Finally, progerinin (Code No. SLC-D011), an optimized drug candidate, was obtained through chemical screening. The efficiency of a therapeutic approach using progerinin in structurally blocking the aberrant interaction of progerin and lamin A has been recently proven in vitro on HGPS patients’ fibroblasts and in vivo on LmnaG609G mouse model. Selective binding of progerinin to progerin block the interaction of progerin with lamin A leading to normalization of the nuclear membrane (Figure 2). Moreover, progerinin is more stable than previous one and more effective in HGPS models (Kang et al, submitted). Basis of our mechanism studies, we have obtained the Orphan Drug Designation (ODD) from FDA that would be helpful for further progression.
Preparation for clinical trials
Safety pharmacology studies have been implemented for future clinical trials. Progeirnin showed high protein binding in CD-1 mouse, SD rat, beagle dog, cynomolgus monkey and human plasma and stable in plasma of all species. Progerinin demonstrated high permeability across the Caco-2 cell monolayer but not a responsive substrate for efflux transporters including P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP).
Progerinin was rapidly metabolized in rat and human liver mirosomes, moderately metabolized in mouse and monkey, and stable in dog liver microsomes. Progerinin was rapidly metabolized in 5 species of hepatocytes. Progerinin inhibited cytochrome P450 (CYP) 2C8, 2C9, 2C19, and 3A4/5-mediated testosterone 6β-hydroxylation activities reversibly as a competitive inhibitor and inhibited CYP2D6 reversibly in a mixed mechanism with noncompetitive inhibition as predominant inhibition mechanism. CYP3A4/5 were major enzymes contributing to progeirnin metabolism in human liver microsomes.
The safety of progerinin has been undertaken in oral (p.o.) toxicity studies and in genotoxicity tests. Progerinin was generally well tolerated in single and repeat-dose toxicity studies conducted in rats and dogs. Progerinin, administered repeatedly to rats and dogs for four weeks, showed no toxicologically significant changes, and the target organ was not identified. Toxicokinetics (TK) parameters showed that no significant changes in systemic exposure were observed across any dose level without sex differences. As such, the no observed adverse effect level (NOAEL) was considered to be 500 mg/kg per day for rats and 300 mg/kg per day in dogs, respectively. Bacterial reverse mutation assay, chromosome aberration test, and mammalian bone marrow micronucleus assay were conducted to assess the genotoxic potential of progerinin; results from all assays were negative. Progeinin has not been assessed in the clinical setting and currently preparing IND submission for Phase 1 clinical trials. The first in human study of progerinin will begin in the near future. For this, progerinin is formulated as a nanosuspension supplied as 100 mg/ml strength for oral administration to be used in phase 1 trial.
Conclusion
Active progress in progeria research let to a growing number of treatment strategies. Nevertheless, most of these studies are insufficient in vitro and in vivo preclinical data for transposition to HGPS patients. In fact, the farnesylation of progerin has been target for drug development to prevent aging process in HGPS [15,16]. Because the farnesylated region of progerin cling to the nuclear membrane resulting in nuclear deformation [17,18]. Blocking farnesylation by farnesyltransferase inhibitors (FTIs) in HGPS patients’ derived cells restores nuclear architecture, cell proliferation, and heterochromatin organization [19,20]. Furthermore, treatment with FTIs in Zmpste24-deficient mice enhances growth, body weight, and lifespan and relieves aging symptoms such as osteoporosis [21]. However, Zmpste24-deficient mice are not fairly related to classical HGPS. Human progeria-like Lmna G609G mouse models are suitable for HGPS models. Therefore, we treated FTI in LmnaG609G mice and observed that FTI did not produce promising results in LmnaG609G mouse models (not published). Besides, gene-therapy through CRISPR Cas9 is known as potential treatment for HGPS. However, the gene editing can create unintended, or ‘off-target’ effects [22]. Moreover, inducing progerin clearance through activating autophagy with mTOR inhibitors and reducing progerin downstream effects can be potential therapies for patients with HGPS [23,24]. These therapies show some favorable effects in HGPS models but also caused side effects with toxicity [25,26]. Our recent studies have shown that the binding inhibitors of progerin and lamin A, called progerinin has efficacy in vitro and in vivo on HGPS models. Progerinin alleviates senescence phenotypes of HGPS cells including abnormal cell morphology, and retardation of cell growth and proliferation. This drug also rescues aging features, including sclerosis and cardiovascular defects, and extends the lifespan of human HGPS mouse models. These results suggest that progerinin will have favorable clinical outcomes such as ameliorating cardiovascular pathology, and extending body weights and survival rates in patients with HGPS. Likewise, advanced preclinical toxicity testing in rats and dogs resulted in no side effects and determination of optimal doses, dose frequencies, administration route were performed for future clinical trials. Basis of our preclinical studies and molecular mechanism, progerinin is stable and can be administrated orally. Progerinin, compared to other potential drugs, would be useful for treatment of HGPS without serious adverse effects.
This work was supported by Tech Incubator Program for Startups (TIPS) from Korean Ministry of SMEs and Startups.
- M Eriksson, WT Brown, LB Gordon, MW Glynn, J Singer, L Scott, et al. (2003) Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford Progeria Syndrome. Nature 423: 293-298. Link: https://bit.ly/3dE94oY
- S Gonzalo, R Kreienkamp, P Askjaer (2007) Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res Reviews 33: 18-29. Link: https://bit.ly/2Ya8N6w
- RC Hennekam (2006) Hutchinson–Gilford progeria syndrome: review of the phenotype. Am J Med Gene Part A 140: 2603-2624. Link: https://bit.ly/3cDqil0
- MA Merideth, LB Gordon, S Clauss, V Sachdev, AC Smith, MB Perry, et al. (2008) Phenotype and course of Hutchinson–Gilford progeria syndrome. N Engl J Med 358: 592-604. Link: https://bit.ly/3dD2AXx
- JI Rodríguez, P Pérez‐Alonso, R Funes, J Pérez‐Rodríguez (1999) Lethal neonatal Hutchinson‐Gilford progeria syndrome. Am J Med Genet 82: 242-248. Link: https://bit.ly/30pLEjx
- PJ Gillar, CI Kaye, JW McCourt (1991) Progressive early dermatologic changes in Hutchinson‐Gilford Progeria syndrome. Pediatr Dermatol 8: 199-206. Link: https://bit.ly/3dE9rzS
- A De Sandre-Giovannoli, R Bernard, P Cau, C Navarro, J Amiel, I Boccaccio, et al. (2003)Lamin a truncation in Hutchinson-Gilford progeria. Science 300: 2055. Link: https://bit.ly/2UdGEdE
- A Mattout, T Dechat, SA Adam, RD Goldman, Y Gruenbaum (2006) Nuclear lamins, diseases and aging. Curr Opin Cell Biol 18: 335-341. Link: https://bit.ly/30doUCV
- RD Goldman, DK Shumaker, MR Erdos, M Eriksson, AE Goldman, et al. (2004) Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson–Gilford progeria syndrome. Proceedings of the National Academy of Sciences 101: 8963-8968. Link: https://bit.ly/2XBFCKt
- AD Basso, P Kirschmeier, WR Bishop (2006) Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res 47: 15-31. Link: https://bit.ly/373UILW
- LB Gordon, ME Kleinman, J Massaro, RB D’Agostino Sr, H Shappell, et al. (2016) Clinical trial of the protein farnesylation inhibitors lonafarnib, pravastatin, and zoledronic acid in children with Hutchinson-Gilford progeria syndrome. Circulation 134: 114-125. Link: https://bit.ly/375O4oq
- N Kubben, JW Voncken, J Demmers, C Calis, G van Almen, et al. (2010) Identification of differential protein interactors of lamin A and progerin. Nucleus 1: 513-525. Link: https://bit.ly/30aeQLb
- N Zhong, G Radu, W Ju, WT Brown (2005) Novel progerin-interactive partner proteins hnRNP E1, EGF, Mel 18, and UBC9 interact with lamin A/C. Biochem Biophys Res Commun. 338: 855-861. Link: https://bit.ly/30cEkaB
- S Lee, Y Jung, M Yoon, S Kang, A Oh, et al. (2016) Interruption of progerin–lamin A/C binding ameliorates Hutchinson-Gilford progeria syndrome phenotype. J Clin Invest 126:3879-3893. Link: https://bit.ly/3h2Zdv8
- LG Fong, D Frost, M Meta, X Qiao, SH Yang, C et al. (2006) A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 311: 1621-1623. Link: https://bit.ly/376xrcz
- SH Yang, MO Bergo, JI Toth, X Qiao, Y Hu, et al. (2005) Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc Nat .Acad Sci U.S.A. 102: 10291-10296. Link: https://bit.ly/3gZfu45
- Y Wang, C Ӧstlund, JC Choi, TC Swayne, GG Gundersen, E, et al. (2012) Blocking farnesylation of the prelamin A variant in Hutchinson-Gilford progeria syndrome alters the distribution of A-type lamins. Nucleus 3:452-462. Link: https://bit.ly/3dEmDVs
- X Lu, K Djabali (2018) Autophagic removal of farnesylated carboxy-terminal lamin peptides, Cells. 7: 33. Link: https://bit.ly/3dF3cLY
- D Gabriel, DD Shafry, LB Gordon, K Djabali (2017) Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson-Gilford progeria fibroblasts. Oncotarget 8: 64809-64826. Link: https://bit.ly/3eZOVdj
- IS Mehta, CH Eskiw, HD Arican, IR Kill, JM Bridger (2011) Farnesyltransferase inhibitor treatment restores chromosome territory positions and active chromosome dynamics in Hutchinson-Gilford progeria syndrome cells. Genome Biol 12: R74. https://bit.ly/3eXRlsC
- K Harhouri, D Frankel, C Bartoli, P Roll, A De Sandre-Giovannoli, et al. (2018) An overview of treatment strategies for Hutchinson-Gilford Progeria syndrome. Nucleus 9: 246-257. Link: https://bit.ly/2MypkMb
- L You, R Tong, M Li, Y Liu, J Xue, et al. (2019) Advancements and obstacles of CRISPR-Cas9 technology in translational research, Molecular Therapy-Methods & Clinical Development 13: 359-370. Link: https://bit.ly/2XC6OZF
- SM Guilbert, D Cardoso, N Lévy, A Muchir, X Nissan (2020) Hutchinson-Gilford progeria syndrome: Rejuvenating old drugs to fight accelerated ageing. Methods. Link: https://bit.ly/3cBJ8ZJ
- MA Rahman, FI Jahan, J Bashira, M Akter, F Islam, et al. (2019) Scholars Academic J Pharmacy.
- F Chiarini, C Evangelisti, V Cenni, A Fazio, F Paganelli, et al. (2019) The cutting edge: the role of mTOR signaling in laminopathies. Int J Molecul sci 20: 847. Link: https://bit.ly/30ekmw4
- R Kreienkamp, S Gonzalo (2020) Metabolic Dysfunction in Hutchinson–Gilford Progeria Syndrome. Cells 9: 395. Link: https://bit.ly/3eTn2TX
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
